The future of gene therapy
Farmazian doktorea. Biofarmazia, Farmakozinetika eta Farmazia-teknologiako irakasle kolaboratzailea
Farmazia Fakultatea UPV-EHU, Vitoria-Gasteiz
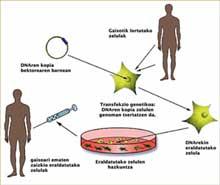
The concept of gene therapy is relatively simple and simple: an appropriate copy of a gene is given to a patient to correct their pathological disorder, i.e., DNA acts as a drug in gene therapy. However, successfully carrying out this concept is not easy. It is very difficult to insert a proper copy of a gene into a specific place in a cell.
Correcting and transferring copies of DNA to appropriate cells is done in many cases with viruses. For many viruses, this is the way to cause infection: they incorporate genetic information into the victim's cellular DNA to reproduce. In gene therapy, researchers wanted to take advantage of this intrinsic capacity of certain viruses, but in this case to introduce a curative gene into the DNA of the ‘victim’.
In the early 1990s, due to promising laboratory animal trials, several research groups in Paris, London and Milan began conducting gene therapy trials with humans. The goal was to cure the evil of children known as bubble children. These children should live completely isolated from the environment, because due to an immunodeficiency, the cells involved in the body's immune response do not develop properly. Consequently, any common disease can cause death.
Unfortunately there are no medicines to cure this serious disease. The only treatment would be bone marrow transplantation from donors with the same genetic characteristics. However, the effectiveness rate of this last solution is low (30%) and presents a risk of severe immune alterations.
XX. A total of 18 children participated in clinical trials in the late 20th century. Gene therapy trials were conducted with retroviruses, some of which integrate their genetic information into the victim's DNA. The results were very satisfactory from the beginning. 17 children recovered immune system function and are alive and healthy today.
As a result of these results, in addition to verifying the therapeutic potential of gene therapy, new clinical trials were launched and the creation of biotechnology industries was promoted.
Unfortunately, the two children who participated in the French trial developed a leukaemia disease in late 2002 and suddenly the optimism generated around this technology became concern and nervousness. In fact, many remembered the death of a young man in another essay in 1999.
During this period, viral particle overdose and subsequent violent immune response were responsible for the tragic outcome. The appearance of leukemia was a very serious news, on the one hand, because in all the research carried out until then with laboratory animals there had never been knowledge of such an alteration and, on the other hand, the safety of gene therapy was again questioned.
The short-term consequences were very harsh for the future of gene therapy. It should be noted that ensuring patient safety is the first requirement that any new treatment must meet. Health regulatory associations in several countries immediately banned more clinical trials and some gene therapy companies abandoned retroviruses and began working with other viruses that offered greater safety.
Successes and failures of gene therapy
There is no doubt that gene therapy is effective in treating diseases that cause immunodeficiency. The cells obtained from the bone marrow of patients are taken and transformed with viruses that have incorporated the appropriate copies of DNA. Once DNA copies have been integrated into the genomes of cells, mature and functional cells are obtained that are injected into patients to cure them. The appearance of leukemia indicated that something had gone wrong in that process, but what?
The answer was found by von Kalle’s research team, a process known as “mutagenic vaccination.” In this process, when integrated into the cell genome, retrovirus changed undesirable genes, including the LMO2 gene that participates in childhood cancers. This showed that the phenomenon to be controlled is the exact place in which viruses integrate into the genomes of cells to prevent these alterations from being repeated in the future. But how can such precision be achieved if the patient is given millions of cured cells in the laboratory?
Looking for solutions
All experts believe that the future of gene therapy should be based on safety and efficacy. To achieve this, more backup vectors are needed to integrate DNA copy only into desired cells, with security, efficiency and accuracy.
Some people think that transporters that do not enter the genome may be a good option, but others believe that the only viruses that are useful for producing long-term changes in cells are retroviruses. For safety, viruses that carry ‘suicide genes’ can also be used. These viruses, if integrated into inadequate cells or multiplied uncontrollably, can be inactivated after administering a drug to the patient.
On the other hand, in recent years the so-called first generation gene expression systems have been optimized. In these systems, transporters respond to specific stimuli: medicines, heat, radiation, amount of glucose or oxygen, etc.
For example, the gene expression system integrated in a patient with diabetes will aim to produce insulin, but this production must be very controlled to avoid hyperglycemia and hypoglycemia. With these new systems you can design a system that produces more or less insulin depending on the level of glucose, so that insulin is only released when the patient needs it and also at the necessary dose.
A similar treatment system for anemia, which produces erythropoietin (EPO), is also being investigated depending on the level of oxygen in the blood.
In addition, it is essential to improve the quality controls available for the study of cells transformed with DNA. The development of appropriate molecular and genetic techniques would allow the analysis of samples of modified cell beams in each shift. It would be best to study all the cells, because each has its own characteristics, but today it seems impossible, since it takes a long time and the trials would never end.
Recovering lost confidence will take time to recover, but over time, gene therapy patients have produced more efficient and, above all, safer systems. Although much work remains to be done, it can be said that gene therapy has not yet written its last chapter.
BIBLIOGRAPHY
- Orive, G. and others ‘Gene therapy, from
hope to reality’
Elhuyar Zientzia eta Teknika, 170:27-32 (2001).
Cavazzana-Calvo, M. and others ‘Gene
therapy of human severe combined immunodeficiency (SCID)-X1 disease’
Science, 288:669-672 (2000).
Check, E.
‘Second cancer case halts gene-therapy trials’
Nature, 421:305 (2003).