Cells also have post offices: coroideremia
We began in January 2014, as promised to the Olentzero, with the aim that the Association of Affected by Coroideremia CHM, Protein Rab Escort (REP1) Type 1, Rab27, RabGTPase or Prenilation is more known. Choroideremia is a genetic disease that suffers one in every 50,000 inhabitants. In the choroidal region, the Rep1 protein gene, known as CHM, is affected by a mutation. Therefore, patients do not synthesize Rep1 functional proteins.
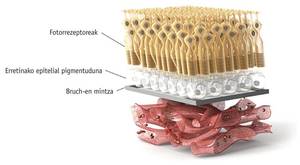
Since the CHM gene is found on the sexual X chromosome and has an underlying character, choroideremia is mostly suffered by men, although it also affects individual women. The main damage is seen in the eyes of the mutated CHMs, in the retina itself and, as its name suggests, in the coronation. It is evident the degeneration of the tissues of the retina that shows the deep image of the eye. In the choroidal area, the choroid and retina begin around the eyeball and degenerate into the fobear, creating blindness in the same direction, from outside into, following the pattern of the tunnel effect. At the same time, patients lose their sensitivity to light and have vision problems when there is some darkness, which is known as night blindness.
Rab Geranilgeranil transferase (RGGTase) is an enzyme like any other enzyme of protein nature, active zone, etc. What, then, is so important to you as in this article?
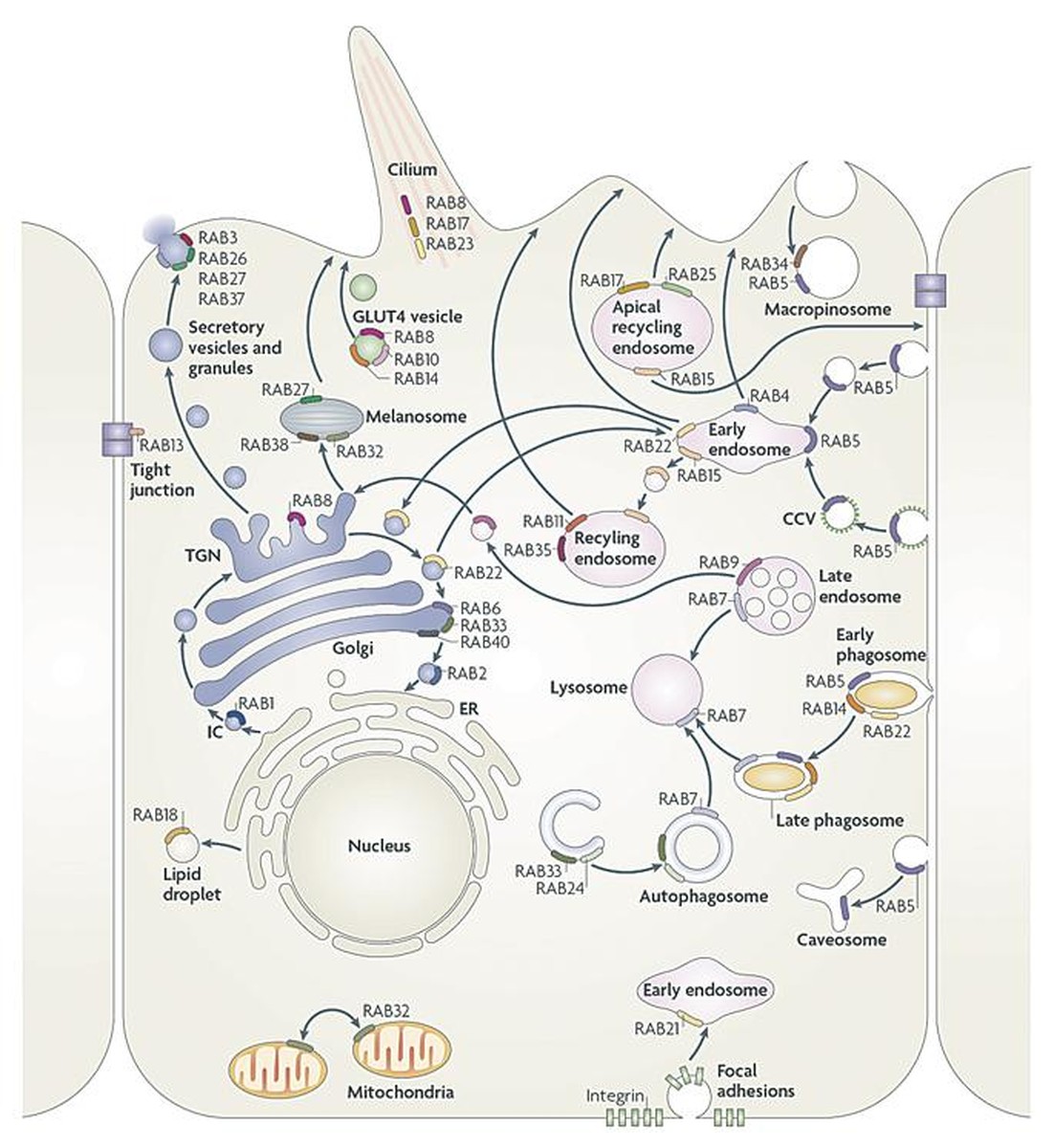
If we look inside the cell we see several molecules as if they did not reach here. Some are attached to the cytoskeleton; others, as if they were letters of mail, are transported in jijones surrounded by membranes until they find their way. This type of vesicle transport is performed between the different compartments of the eukaryotic cell, such as the Golgi apparatus, the endoplasmic network, vacuoles, melanocytes and the outer membrane.
But how do you know where to go? Who sets the direction and seal to the envelopes? For this we have Rab proteins (Rab GTPases), small proteins of a single polypeptide chain. The direction marked by the rab proteins depends on the state GTP(on)-GDP(off). There are more than 60 different rabs, each identified by a number, and in the language of the cell each indicates a different direction. For example, Rab 27 marks melanocytes. Therefore, when a transport vesicle is formed between the cell compartments, a Rab protein must be associated to know where it should be transported. As a hydrophobic protein, the rab is attached to the hydrophobic part of the vesicular membrane. This is where the RGGTasa enzyme does its job, sticking the envelopes in the right direction so that everyone can go to their site and perform their function correctly. In other words, the enzyme RGGTase specializes in protein prenylation and presses RabGTPases. It is called prenylation or geranilgeranilation to the association of the geranil group to proteins, that is, to the cysteine amino acid of the terminal carboxyl part. This 20-carbon isoprenoid, RGGTase, modifies the nature of the soluble Rab protein itself. Binds two hydrophobic tails to the hydrophilic protein Rab to anchor inside the double layer of the membrane.
The chaperone protein Rep1(Rab escort protein 1) to RGGTasa will help to stick the correct direction to the envelopes. The Rep1 txaperon will collect and mold the Rab protein until the RGGTase enzyme does its job and adheres to the Rab protein. We also have the txaperon Rep2, which performs a work similar to that of Rep1, but because of its less affinity with the enzyme, is not able to do the same as Rep1. However, Rep proteins are necessary, because if there are no proteins or if you suffer damage to them, Rab proteins and vesicles accumulate in the cytosol. Consequently, certain functions that depend on exocytosis remain unperformed.
As mentioned above, the consequences of the lack of mechanism of Rep and Rab proteins in choroideremia are serious, for example, blindness. However, to better understand why blindness develops, it is necessary to better explain the molecular mechanism. Rep1 is essential to prenilar the protein Rab27 in the cells of the choroid. Because in the cytoplasm of the eye cells of patients with choroideremia Rab27 is not prenylated, melanocytes are left without markers. This lack generates two main problems. On the one hand, there is no exocytosis of the residues necessary for the maintenance function of the choroid, and the cells die due to its high degree of toxicity. On the other hand, since properly formed melanocytes cannot secrete melanin, the retina remains unprotected and degenerates. Photoreceptor cells of the retina require a continuous renewal of the membrane of the discs by exocytosis. Membrane renewal is essential to maintain functional rhodopsin molecules that make light quinada a nerve impulse. In fact, the lack of functionality of rhodopsin molecules is the main cause of glare in crowned people.
Lately there have been some attempts to cure choroideremia. In January, the first clinical trials with gene therapy were conducted in the UK at the University of Oxford. Dr. Robert E McLaren transferred the CHM gene to 6 patients using gene therapy with viruses. If this therapy to cure retinopathy of choroideremia is effective and safe, it would be a hope for other patients suffering from retinal degeneration.
Despite our great hope in gene therapy, we cannot rule out other therapeutic strategies. In this sense, researchers awarded the 2013 Nobel Prize in Medicine deserve special mention. Researchers James Rotham, Randy Schekman and Thomas Südhof were awarded for their research on cell transport. These Nobel prizes investigated proteins Rep1 and RabGTPase. Deepening research on these proteins would increase knowledge about the transport and secretion of enzymes, hormones, neurotransmitters, etc. and would open the doors to new therapeutic strategies for the choroidal zone. Until we find a medicine that cures choroideremia, we should also think about the possibility of therapies that, in a shorter time, would be useful for these patients who are becoming blind. For example, molecules that have demonstrated the inertisation capacity of retinal cells can be investigated. However, to demonstrate the effectiveness of all these strategies proposed in this article, it is necessary to develop valid models to investigate choroideremia and, if possible, to avoid the use of animals, which are in vitro models. Basic research staff also need to join clinicians and collaborate to benefit patients.
Instead of giving in to this situation, in February 2012 we decided to create the Association of People Affected by Coroideremia. Since the creation of the Association, at the state level, we have contacted 17 families with choroid and we have 61 partners. In addition, researchers are constantly struggling to raise funds to develop treatment based on gene therapy before our children and associates lose sight of it. In this sense, in collaboration with the IES of Ondarroa and the research group NanoBioCel of the UPV/EHU, we have organized the first research days on the choroidal area that will take place on 16 and 17 September at the Faculty of Pharmacy of the Campus of Álava. These scientific days will work the choroideremia from six points of view: clinical, pathophysiology and genetic molecular characterization, neuroprotective therapy, gene therapy, cell therapy, in vitro and in vivo models and clinical trials.