Our body, a powerful weapon against cancer
Omaetxebarria Ibarra, Miren Josu
EHUko Biokimika eta Biologia Molekularra Saileko irakaslea eta ikertzailea
Cells are basic parts of life; the better we know the structure, functioning and interactions between them and their environment, the more we see them as machines close to perfection. These cells are able to divide in thousands of occasions, and although the process of division, complex, is extremely accurate, occasionally errors can occur. Cells have many mechanisms to remedy these errors, but, although rarely, in some cases these errors are irreparable. In these cases, cells can divide uncontrollably and make them immortal, i.e., develop cancer.
Traditionally, cancer has been attacked by chemical and/or radioactive agents. These therapies, in addition to damaging the DNA of fast-growing cancer cells, also affect the DNA of healthy cells, which requires managing their toxicity and unpleasant side effects during therapy. Thus, in recent years the scientific community and the medical community are taking important steps in the development of less aggressive, more effective and specific therapies. In this sense, immunotherapy is the most outstanding. The path taken by immunotherapy is so encouraging that in 2013 the journal Science called it annual progress. Immunotherapy opens a whole new way to treat cancer, where instead of having the tumor itself, the immune system is sought. When we talk about tumor immunity we talk about the efficacy of host antitumor immunity. To ensure this, it is imperative that different types of immune system cells act in a coordinated manner, as the immune response is a complex consequence of multiple interrelated processes.
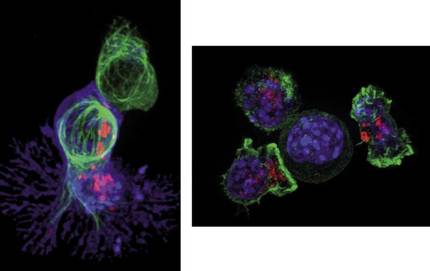
In this complexity, T cells or T lymphocytes play an important role in the development of the immune response. Millions of T cells travel through the blood at all times observing the presence of foreign elements. A spoonful of blood can contain about 5 million T cells, as small as many. They have an average length of 10 micrometers, a tenth of the thickness of a human hair. In healthy people, T cells remain during sleep, but when a foreign component or cell, such as a cancer cell, is detected, T cells begin to divide in order to fight and destroy it. Figure 2 shows what happens when a T cell detects a cancer cell. Once the T cell (in green) binds to the cancer cell (in blue), the former incorporates “poisonous” proteins (red dots) known as cytotoxins. As a result, the cancer cell dies and T lymphocyte is willing to look for its next victim.
As discussed above, before destroying the foreign element, T lymphocytes are fragmented to increase in number and make the response more effective. The small molecule called interleucleukine 2 (IL-2), synthesized by the immune system itself, provides the starting signal for the division of T cells. Given the ability of IL-2 to reproduce T cells, it was used to treat a woman with skin cancer three decades ago. It was a woman who had been subjected to a series of treatments that had not meant any improvement. Treatment with IL-2 showed massive cell death at the edge of the tumor. Tumors contracted within two months and no signs of cancer were detected after a few months. In the next 29 years the woman has not developed any other cancer. This was the first test of the efficacy of IL-2 treatment and therefore of the patient's immune system's ability to destroy tumors. Since 1992, with the approval of the Food and Drug Administration (FDA), IL-2 is the usual treatment for skin cancer and renal cancer.
Tricks used by tumors to prevent the immune system
But why should T cell proliferation be encouraged? Are they unable to recognize and attack cancer cells? Unfortunately the answer is negative, and to explain it we must look at the peculiarities of the tumor micromedium. Cancer will attempt to escape or alter the host's immune response to ensure its survival and development, causing, among other things, inhibition of key T cells. Although it seems contradictory, in tumors, in addition to T lymphocytes, there are numerous cells in the patient's immune system, but due to the special conditions of the tumor's microenvironment, the activity of these cells is difficult. For example, in tumors, the number of molecules that secrete the cancer cells themselves and that hinder or cause death of immune cells (tgf-beta, IL-10, prostaglandin E2, etc.) is enormous, while the amount of oxygen present in the tumor itself is scarce and under these conditions tumor cells develop mechanisms of life, but immune cells are not able to do so. In addition, in this attempt to escape the immune response, cancer cells will alter the molecular composition of their surface, becoming invisible to the immune system. If this were not enough, cancer cells also develop the ability to block immune cell activity (Figure 3). All of these mechanisms are believed to work together to limit the immune system's ability to fight cancer itself. Therefore, overcoming all these mechanisms is essential for the development of more effective immunotherapy for the elimination of cancer. This is the way we are working today: the manipulation of the activity of immune cells to overcome possible adverse conditions in the microenvironment of the tumor and destroy the tumor. Among the most sophisticated approaches are the use of blocking antibodies against certain T-cell antigens and the use of genetically modified T cells. These two main strategies have been recognized in 2013 by the aforementioned journal Science.
The individual, the most powerful weapon to attack cancer
As already mentioned, immune cells are not able to eliminate the tumor, largely because cancer cells block their activity by one way or another. Immune cells present in tumors, including T lymphocytes, often indicate special proteins or receptors on the surface. In turn, cancer cells in the tumor may represent the molecules that are associated with these receptors. In these cases, interactions between cancer cells and T lymphocytes occur that will block the activity of T lymphocytes (3A. Image).
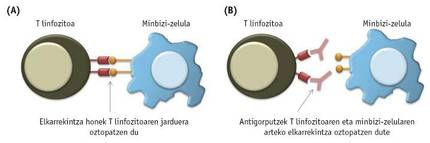
To avoid these harmful interactions between cancer cells and T cells, several antibodies have been synthesized in recent years (3B. Image). Tests so far have shown that the most successful are antibodies associated with the PD-1 and CTL4 receptors of T lymphocytes. In 2011, the FDA approved a drug called ipilimumab that blocks CTLA-4 receptors to treat skin cancer. Although initially the results obtained in both ipilimumab and IL-2 were similar, it has subsequently been observed that in patients treated with ipilimumab treatment is longer. Opdivo or nibolumab, a drug that blocks the PD-1 receptor of T lymphocytes, was approved by the FDA in 2014 to treat melanoma, and one year later was accepted to fight lung cancer.
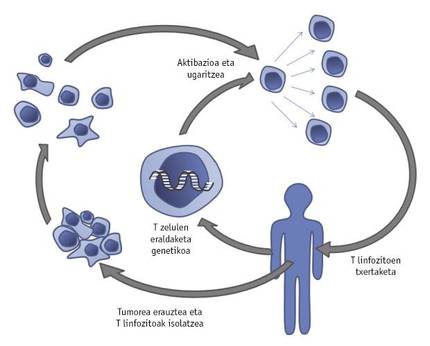
The development of an innovative strategy, called adoptive T-cell transfer, has also given a major boost to the fight against cancer. This therapy involves isolating T cells from the tumor extracted from the patient. These T cells will be genetically manipulated by introducing new genes or transforming their own to grow and act more effectively in the tumor micromedium. After promoting the growth of improved T lymphocytes with IL-2, the patient will be reinstated (Figure 4).
Proof of this is done by a group of researchers from the American state of Maryland: T cells were genetically modified to represent the IL-12 that causes inflammation. Once inserted into the tumor, modified T cells secrete IL-12, which prevents the activity of cancer cells that interfere with the immune response. Taking into account the promising results obtained in mouse experiments, the first clinical trials with genetically manipulated T cells indicating IL-12, patients with melanoma, are being conducted. Another problem to overcome is the excess mortality of tumor-typical T lymphocytes to ensure effective immunotherapy. To combat this, T lymphocytes have been genetically modified by the same research team to represent the Bcl-2 molecule that prevents their death. Comparing the T lymphocytes that represent this molecule with the unmanipulated, it was demonstrated that the former are more resistant to death. As mentioned above, in many cases the immune response in the tumor micromedium is obstructed by the inability of immune cells to know the tumor and therefore combat it. In this sense, it has been shown that T cells present in tumors may be genetically manipulated permanently to express exciting molecules and, consequently, that T cells remain activated without detecting the tumor itself and thus destroy the tumor.
The success of this therapy has been remarkable from the beginning, and its effectiveness increases with research.
Adoptive T-cell transfer therapy has recently been shown to be significantly more effective if treated through chemotherapy or radiation therapy to cancer patients before undergoing this treatment. These therapies eliminate the patient's intrinsic lymphocyte population, making introduced genetically modified T cells capable of acting more effectively and for longer. Unfortunately this has disadvantages, there are patients who are not able to advance in this situation without T cells.
There is still much to improve
Although significant advances have been made in both research and medicine in recent years, cancer remains one of the most deadly diseases in the world. Deeper knowledge of immune systems will be essential to develop more effective, specific, sustainable and less aggressive therapies. It will be essential to make combinations between different strategies that allow to adequately face such a heterogeneous disease from the biological point of view.