The genomes of the culprits of malaria in sight

All this information will be used to reveal the best kept secrets of the disease. The final goal is to find new strategies to fight malaria. It must be taken into account that this is a mortal disease. According to data from the World Health Organization, every year 300 million people are seriously ill and die at least one million because of malaria. About 90% of deaths occur in Sub-Saharan Africa and every 30 seconds a child sick from malaria.
The first symptoms of malaria appear between 9 and 14 days after the bite of mosquitoes, and are similar to those of the flu: fever, headache, nausea... If the disease advances, anemia appears and blood vessels that go to the brain and other major organs are blocked. As a result, these organs do not receive blood and, finally, they can die.
Parasite and mosquito

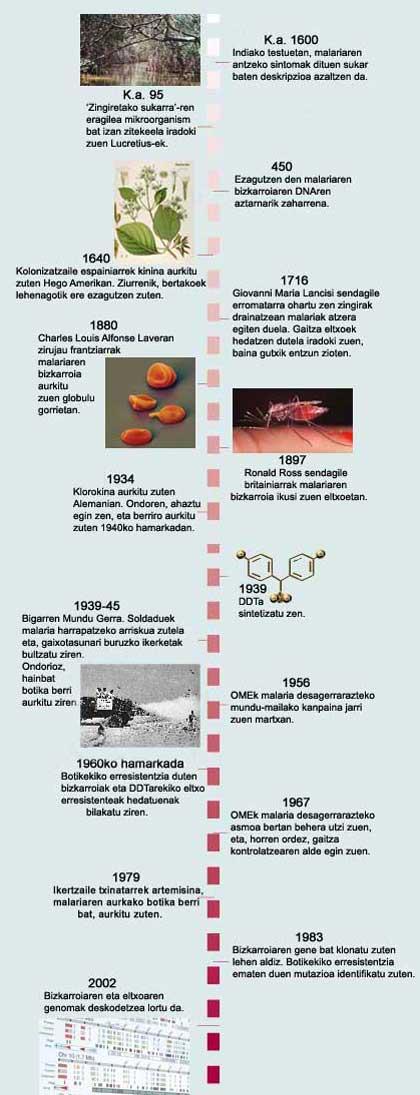
Malaria has been known for a long time, but until just over a century ago they did not know what causes the disease. It was formerly considered that the origin of the disease was that of the shelled marshes, hence its name (‘evil aria’ or bad air). In 1880, scientists discovered the true culprit of malaria: Plasmodium single-cell parasite that infects the human liver and red blood cells. Later it was discovered that the parasite is transmitted from one person to another by the puncture of the female mosquitoes of the genus Anopheles.
In humans, malaria is caused by four species of Plasmodium: P. malariae, P. vivax, P. ovale and P. falciparum . The latter is responsible for the most serious type of malaria. In all of them, the vector is the Anopheles mosquito, through which it is essential to pass the parasite so that the cycle advances and the parasite becomes a contaminant of the human being. In fact, Plasmodium has a complex cycle and not all stages were known until 1948.
Genome of the parasite Plasmodium falciparum

One more step has now been taken: within an ambitious malaria research project, three biochemical groups have developed a project to decode the genome of the microorganism P. falciparum. A team belongs to the English laboratory Sanger Centre (Cambridge) and has decoded nine chromosomes. The rest of the chromosomes have been decoded by two groups from the United States, the TBAJO Institute (Maryland) and the Stanford University (California).
At the same time, the genome of another similar microorganism has been decoded, the Plasmodiun yoelii yoelii, responsible for malaria in rats; compared both, scientists will know what makes a microorganism harmful to man and the other not.
Scientists have spent a lot of time decoding the genome of P. falciparum, which is very significant. It can be thought that completely decoding the genome of any organism is and is so, but due to its size they have taken too long.
P. falciparum's DNA consists of 23 million base pairs, divided into 14 chromosomes. The data are only numbers, although, for example, the genome of the Drosophila melanogaster fly, with 120 million base pairs, was decoded in less than a year. However, the genome project of this microorganism was launched in 1996, and is an obvious consequence of the difficulties that have been found to require such a long term.

The origin of these difficulties lies in the components of the genome, in fact the methodology that has been successful with other genomes is not appropriate for the study of Plasmodium falciparum. In fact, the bases that use DNA are four, G, C, T and A, but this microorganism has very long sequences composed only by bases A and T. To decode the genome, the DNA is broken into small parts, each piece is decoded and computerized to search for the original sequence. This last step is complicated when instead of four bases only two participate.
In the chromosomal structures of this microorganism, a series of peculiar characteristics have also been found. For example, the structure of extreme telomeres is very complex, which, according to scientists, facilitates the passage of mutations, many of them occur in those areas of the genome. Therefore, Plasmodium are quite variable microorganisms, which prevents progress in the investigation of malaria.
The mystery of metabolites
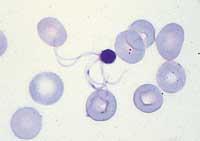
In addition to the difficulties of decoding, these sequences generate problems of interpretation. We know that such sequences do not encode gender, but it may be necessary to rethink them.
During research, software has been used to detect where genes are found (with intron), but these programs are not accurate and their criteria for identifying a gene are insufficient or correct. In the case of the human genome there was also the same problem, since it is not yet possible to say how many genes the human being has.
The strategy currently used is the comparison with known genes. For this purpose, genes from other similar organisms are used. Of course, this is not a success, since it meets the genetic characteristics of each species. The genome of Plasmodium falciparum, in addition, has provoked great surprises. For example, typical proteins that "manage" the basic molecules that transport energy have not been exposed in these studies, either ATP or NADH.

In addition, few transport proteins have been found. However, researchers have detected at least one sign of mitochondrial activity and have identified many genes related to apicoplastes (areas of fatty acid synthesis).
What does all this mean? Are we facing a new type of metabolism? Or is the methodology for finding proteins still very delayed?
The genome of the mosquito also decoded
Although P. falciparum is the main culprit of malaria, in order to infect human beings it is essential the participation of the female mosquitoes Anopheles, since the parasite occupies inside one of the stages of its cycle.
Numerous international researchers participated in the decoding of the mosquito genome. Led by Robert A, by Celera Genomics. It has been Holt, and among all have managed to analyze the genome of the mosquito Anopheles gambiae. For this purpose the shotgun method has been used, that is to say, several parts of the genome have been randomly sequenced and the ends that overlap have been joined. Finally, a genome of 278 million nucleotides, basic units of DNA, has been obtained.
When the female mosquitoes take human blood, some proteins and blood lipids go to the ovaries and help develop eggs in 2-3 days. After laying the eggs, he returns to look for a guest to catch his blood, make digestion and develop and lay the eggs.
The components that form when digesting the blood cause some genes to be activated and others to be inactivated. To find out what these genes are, the parts of the DNA that encode the genes have been analyzed, both in the female mosquitoes that took blood and those that did not acquire blood, and the results have been compared.
Other researchers have focused on the mobile parts of the genome. These parts are called transposon and have the ability to locate in the genome anywhere. So, sometimes they are out and others inside, and can appear anywhere. As a consequence, logically, variability increases enormously. Well, this type of fragments represent 16% of the genome of the mosquito. In addition, the genes that encode the enzyme that divides DNA have been identified.
After all this, what?
Now that is the main question. Without a doubt, a tremendous job has been done, but then we will have to know how to interpret and use the information. The knowledge of the genes allows to identify the destinations of the new drugs and there have arisen many possibilities.

For example, although they have not been able to understand the metabolism of the parasite P. falciparum, they may be able to design new strategies against it. In fact, five proteins have been identified that participate in feeding vacuoles that could be blocked by specific inhibitors.
Another way would be to prevent the parasite from entering the red blood cells. The proteins present on the surface of the parasite help to escape, so if these proteins are known, new drugs can be designed.
As for the mosquito genome, researchers have considered that there is more than one focus of insecticides or vaccines. In addition, they consider that the moving parts of DNA can be used to introduce new genes into the mosquito genome, for example, to introduce into the mosquito some gene that breaks the parasite cycle. The latter can also be used as markers to differentiate mosquito populations from the same species, since some populations are better transmitters of malaria or more resistant to insecticides.
They have also analysed how the mosquito addresses man. Apparently, the mosquito has the capacity to recognize the smell of the human being, and with it choose who to arrive. Now the smell receptors of A. gambiae have been identified; to avoid mosquito sting it would be enough to block them.
In addition, several scientists propose to genetically transform the mosquito to make it unable to transmit the disease. Already these mosquitoes have been achieved and some propose their release in nature to replace the populations of common mosquitoes. Before, however, it is necessary to deepen the ecology of the mosquito, on which one of the groups of researchers of the project has acted. Others have treated the immune aspect, etc.
In any case, because half is believed to be corrupt and there is still much work to be done, it is better to be prudent. According to the researchers themselves, during their work, they had a doubt: what is the best way to fight malaria? High level genetic projects? Conventional public health surveillance programs? The solution can be to use both pathways. We must not forget the XX. Until the mid-twentieth century malaria was much more widespread than at present and was common in many temperate countries, including in Euskal Herria.
The installation of basic hygienic services and sewers in some areas has allowed to control malaria by eliminating mosquito breeding zones. In many countries, however, they still do not have such advances; in addition, they have a temperature and humidity between 20-30ºC, that is, an ideal climate for the reproduction of the mosquito. To see if the new options opened give them a solution.
Cycle of malaria
When the female Eltxo approaches a person, she introduces the initial forms of her parasite Plasmodium, sporozoites. Sporozoites escape from the immune system and reach the liver through blood circulation. There, each sporozoite creates a special structure called schizonte, each of which contributes thousands of merozoites. Thus, for 12 days, a liver cell may contain thousands of marozoites or young parasites. When schizophone arrives, merozoites are released into the blood and rapidly enter red blood cells.
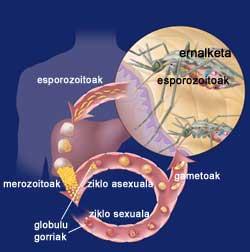
Within the red blood cells, the parasites grow in two ways, since they can have an asexual sexual cycle. In the sexual cycle, female and male gametocytes are formed. They die in the blood and when a female mosquita arrives they meet in the stomach of the mosquito. On the wall of the stomach are formed oocysts that after a few days give rise to a large number of sporozoites. Sporozoites are collected in the salivary gland of the mosquito and are prepared to be introduced into the inside of the host through the next puncture.
On the other hand, in the asexual cycle, the parasites develop in the red blood cells. For their development, they use the hemoglobin of red blood cells, responsible for the transport of oxygen in the blood. As in the liver cells at the beginning, inside are formed some schizontes rich in merozoites that, when they mature, explode and return to the blood. In passing, red blood cells are destroyed. And much more will be destroyed while the disease is not controlled, as newly released merozoites will contaminate other red blood cells.
Sterile fight against malaria
Many anti-malaria drugs have been tested. Formerly, the quinine, a natural product obtained from the quinine bark, was used very much. But being very toxic, it was discarded. When chloroquin was discovered, a great step forward was taken for its low cost and effectiveness. However, the four Plasmodium species that cause malaria in humans have developed resistance and now researchers are looking for a drug to replace chloroquin. By decoding the parasite genome they discover how the parasite has achieved this resistance and that of other drugs.

To end the mosquito, different strategies have been used, especially insecticides. Among them, a dangerous DDT contaminant has been used. The mosquito, however, has been able to resist everyone.
It is believed that resistance to insecticides is manifested by two mechanisms: the increase in the expression of the genes that detoxify the insecticide or the mutation of the genes that encode the target proteins of insecticides. Two types of genes have been detected in the genome, as well as variants called SNP in individual nucleotides. In the opinion of Holt, this information can serve to identify targets for developing new insecticides.
Other pathways based on the interruption of the parasite cycle in the host body or on the promotion of the immune system have also been addressed. Many people also have many expectations in the vaccine of researcher Patarroyo. However, there is still no total solution to fight malaria. Hence the interest to know the genetic codes of the mosquito Anopheles and the parasite Plasmodium.