Colloids: what, where and for what
What do ice cream, cement, smoke, beer, cowboy and pesticides have in common? These and other chemicals we use daily are colloids. All colloids are formed by a homogeneous medium and small particles scattered or dispersed in it. Other colloids anyone knows are butter, blood, and paper.
The science of colloids studies chemical systems of intermediate dimensions between micrometers (1 m = 10 -6 m) and nanometers (1 nm = 10 -9 m), i.e. chemical entities from smaller aggregate particles to larger atoms or molecules.
Although, in general, it has a lot to do with physical chemistry and chemistry, the science of colloids is an interdisciplinary research. Colloids are often very complex and studies that are done to understand their characteristics well are not as accurate as scientists desired. Perhaps it is a complex system that is science in different fields, for many years and still is, most schools and universities have not been exposed.
Colloids of different types and sizes
W. Ostwald XX. The first exact classification of colloids is due to the Nobel Prize winning scientist in the early 20th century. For the first time, all colloids were established in dispersion form, and the German Russian scientist decided to classify them by sizes. It considers that dispersions can be classified into three large groups: particles and aggregates greater than 10 -7 m, such as mud; colloidal dispersions of 10 -7 -10 -9 m; and molecular dispersions less than 10 -9 m.
Currently, 2x10 -7 -5x10 -7 m systems are also considered colloids. Therefore, as decided at the beginning of the last century, colloids are larger than water, hydrogen or sugar molecules, but smaller than blood cells and bacteria.
The Nobel Prize Staundiger, instead of doing so by measure, took into account the number of atoms of colloids when sorting them. Staundiger believes that a particle of colloids should contain at least one thousand atoms. A water molecule has three atoms and, of course, it is not a colloid. A sugar molecule that gives the cane plant has 45 atoms, which is also not colloid.
The molecular weight of an organic molecule of a thousand atoms is about 10,000 g/mol, and if they were placed around a sphere they would form a particle of 0.5-2 nm. According to Staundiger, therefore, the colloids should have between 10 3 and 10 9 atoms.
DISPERSION EXTENSION / 10 -9 mQuartz grain diameter 50.000-20,000 mHuman blood cell diameter7500 Bacterium diameter Bacilus Coli 1500Sulfur colloid particle diameter 50-500 Influenza virus dimensions 120Gold colloid particle diameter 1-100 Elemental oxygen dispersion molecule dimensionsMost important colloids: emulsions
As we have seen at the beginning, colloids can be very varied. Perhaps the most used in the industry are emulsions. A society without emulsions would be unimaginable today: many medicines, asphalt, butter, toothpaste, body creams, agricultural products, etc. are emulsions. As a large group, understanding the physical-chemical characteristics of emulsions is of great importance to everyday life.
Emulsions are dispersions between two insoluble, thermodynamically unstable liquids. Normally one of the two liquids is water and the other any other that is not soluble in water, which we usually define as oil. Emulsions can therefore be drops of oil scattered in water (or/or, for example, milk) or drops of water scattered in oil (or, for example, butter). Either or u/o the diameter of the emulsion droplets is about 5-10 m. In our daily lives predominate emulsions or / or, of which will be reported.
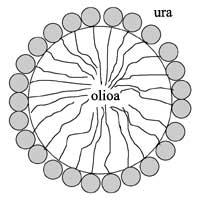
Since both liquids are insoluble, we need some emulsion agent to obtain emulsion drops, i.e. surfactants (SURface ACTive AgeNT). Surfactants are amphilic molecules, that is, they are formed by a hydrophilic head that wants to join the water and a hydrophobic tail that wants to join the oil. Therefore, the hydrophobic tails are oriented towards the drop of oil and the hydrophilic heads towards the water (see drawing 1). Thus, thanks to this unique structure, surfactants have the ability to reduce surface tension and surround the drops. In blends of water oil without surfactant, the surface tension is very high and in a very short time the oil drops would be joined and two completely different phases would remain. In general, used oils are lighter than water, so the oil phase would rise and the top would see the oil layer.

As mentioned above, these dispersions are thermodynamically unstable, that is, when oil, water and surfactants are put together, oil drops are not generated spontaneously. It is necessary to supply energy to the system. The aim of this energy is to increase the oleo-water interface in the oil volume unit. The more drops we make, the smaller they would be, adding the water-oil interface and obtaining a more stable emulsion. The most optional energy source for droplet formation is the agitator, or similar. Therefore, we would be able to prepare an emulsion by selecting the appropriate oils, waters and surfactants and using the necessary energy.
The future of the science of colloids

There are colloids of different size and appearance in nature, since colloids are not only small particles, but chemical entities with different molecular structures and functional groups. It has also been shown that colloid systems are capable of solving problems that cannot be solved with conventional molecules. This principle is not new, since nature has used it in millions of years. One of the most surprising examples is the structure of our hair: keratin molecules are added to different levels until the hair is completed. If we compare the fibers made by nature with those made by man, the former have much better properties. Another illustrative example is our skin. Although the structure is well known (water, collagen and some acid), so far no synthetic material has been found so hard, elastic and soft.
Experts are investigating the synthesis of composites of polymer and colloid composite materials such as skin or other body tissues. It is clear that there is still much work to be done and, undoubtedly, the XXI. One of the branches of science that will most develop in the nineteenth century will be the chemistry of colloids.
Contours PARTICULARESOLOIDE-SISTEMASmists, aerosolSolid Dust, aerosolSolePowder, aerosolévésÃsÃsÃsÃsSolidificaciÃ3n, aerosols, SolemosSolidifiers, Examples of investments.Remarkable historical data
The use of colloids is as old as the first civilizations. To obtain the paint ink, for example, the Egyptians added to the black carbon dispersions in the water the natural gum of the acacia tree or the albumin of the egg white. Gold suspensions were known to medieval alchemists who drank as the elixir of life.

F. Selmi (1843) was the first researcher to systematically study colloids. Despite attempts to dissolve sulfur particles and casein milk proteins, small particle suspensions appeared several times. The first experiments to investigate the main characteristics of colloids were conducted by the English scientist Graham in 1861. On the one hand, he realized that the small ions of salt and sugar molecules moved quickly and that they could easily pass a membrane submerged in water. On the other hand, he discovered that albumin and jelly moved very slowly and that their size was larger than membrane holes. The word “colloid” was his idea; kolla means tail in Greek and discovered that colloids acted as tails. As a result of these first experiments, for the first time the colloids began to be classified by measure.
Perhaps the first most important observations are M. They were made by Faraday in 1857. This British scientist set out for many years to study the optical characteristics of colloids, being the most worked solutions of gold particles. After crossing a golden solution to a ray of light, on the one hand, the solution was whitish. With this observation, Faraday quickly detected that gold particles, like all colloids, scattered light. Another difference between colloids and saline ions (Na+, Cl-) is that ions do not disperse light, but do colloids.
XX. At the beginning of the 20th century many scientists continued to study colloids, and among all perhaps the best known was A. Einstein. This German scientist investigated, among many other things, the size of the colloids, the Brownian movement and rheology. Especially in the latter, he made his most outstanding efforts, managing to explain the viscosity of colloidal dispersions with the equation that bears his name.
In the middle of the last century the first techniques of analysis of the size and molecular weight of the colloids were developed. Techniques were also invented for the study of colloidal dispersion viscosity, x-ray analysis, electrophoresis, etc. Perhaps since the colloids are well known, one of the great inventions has been the electron microscope. The first was developed between 1932 and 1940, being German, British and American pioneers.
The importance of colloids in industry and society
Today, the science of colloids is of great importance in any application. In the means of production used in the oil industry, in medicines, in dairy products, in paints, etc., it is mandatory to understand well the chemistry of colloids.
One of the most important areas of colloids is food chemistry. There are dozens of foods made up of colloids such as milk, butter, cheese, sauces of all kinds, mayonnaise and ice cream. The British Dutch company Unilever sells 500 million liters of ice cream each year.

The characteristics of colloids also have a lot to do with the textile industry. Surface chemistry and tissue wetting are very important in dyeing processes. In photography, printers and ceramics also predominate colloids.
Two important examples are agricultural products and medicine. In the first, soil fertility is associated with the abundance of colloids. The more clays and humus the earth has, the more water it will resist and therefore the plants will have more nutrients. If larger or aggregate particles were used instead of using colloids, the earth would not withstand the water so easily and dry quickly. On the other hand, if we used inorganic salts, they would get into the ground to the bottom and the plants would not be able to sift.
Many other examples can be found in biology. Blood is a complex solution of colloids. Our skin, our muscles and various muscle tissues of the body have gel structures, formed by colloids. Perhaps the most important molecules that we can find in the body of any animal are proteins, which, as we know today, fulfill all the characteristics of colloids in their solutions. In recent years it has been shown that the form of proteins we have in our body is absolutely determinant. For example, collagen and skin bones are building molecules with an elongated shape. Proteins found in blood and milk are globular in shape. It is clear that long molecules are designed to build larger structures, as if they were found in moving fluids, such as blood, they could close any capillary easily.
BIBLIOGRAPHY
- Kaoru Tsujii Surface Activity, Principles, Phenomena and Applications . Academic Press (1998).
- Eric Dickinson An Introduction to Food Colloid s. Oxford Science Publications (1992).
- D. J. Introduction to Colloid and Surface Chemacer. 4th Ed, Butterworth-Heinemann Ltd (1992).





