2006/10/01
224. zenbakia

eu es fr en cat gl
Aparecerá un contenido traducido automáticamente. ¿Deseas continuar?
Un contenu traduit automatiquement apparaîtra. Voulez-vous continuer?
An automatically translated content item will be displayed. Do you want to continue?
Apareixerà un contingut traduït automàticament. Vols continuar?
Aparecerá un contido traducido automaticamente. ¿Desexas continuar?
Treating radioactivity
Text created by automatic translator Elia and has not been subsequently revised by translators.
Elia Elhuyar
By breaking the nucleus of the atom you get energy. Lots of energy. Unfortunately this method is dangerous because the remaining residues emit radiation. These residues can be buried and looked away, but it is not a good solution. What if not? Some physicists believe that waste can be manipulated to reduce radioactivity. Others consider it impossible.
Treating radioactivity
01/10/2006 | Roa Zubia, Guillermo | Elhuyar Zientzia Komunikazioa

(Photo: Archive)
The solution of the problem could be within the core. Some nuclei emit radiation and others do not. If you understand why there can be some way to manipulate the core.
In the nucleus there are protons and neutrons. For chemicals, protons are the most important; the chemistry of the atom depends on the number of protons, so the names of the elements were placed according to the number of protons they have in the nucleus of the atom. For example, any atom that has 7 protons is nitrogen, regardless of the neutrons it has. But for physicists the number of neutrons is also important. A nitrogen has 6 neutrons or 7 are very different. Both are nitrogen, but they are two isotopes, the 13 nitrogen and the 14 nitrogen, respectively (the element representing the isotopes and the number of particles of the nucleus, the protons plus the neutral ones is named). The first is radioactive and the second is fully stable. The difference is in the number of neutrons, which is important; in short, to know if a certain isotope is radioactive, you have to look at the proportion between protons and neutrons.
In order for the core to be stable, protons need neutrons and also in adequate quantity. Neither too many neutrons nor too few. But how much is that? How many neutrons does the nucleus need not to be radioactive by proton? There is no simple answer. In most small atoms the law of equality is fulfilled: how many protons, several neutrons.
Equality
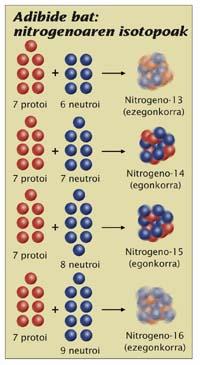
(Photo: G. Roa)
The helium-4 core (2 protons, 2 neutrons) is a good example. Very stable. Moreover, the cores formed by several units of the helium-4 core are also very stable: 12 carbon (6 protons, 6 neutrons, 3 units), 16 oxygen (8 protons, 8 neutrons, 4 units), etc. There are exceptions, for example, the beryllium-8 core (two helium-4 units) is radioactive. However, in general, small nuclei with the same number of protons and neutrons are stable.
However, this trend ends with the calcium-40 nucleus. In larger atoms, the nucleus needs more neutrons than protons to stay stable. For example, the most abundant stable isotope of iron has 26 protons and 30 neutrons. It has 1.15 neutrons per proton. In the case of gold, this ratio is higher, as the only stable isotope is 79 protons and 118 neutrons. That is, to more heavy atom, greater proportion. In the case of bismuth, 83 protons and 126 neutrons, the ratio reaches number 1.52. In atoms larger than bismuth the situation is serious; there are many protons and you cannot put in the nucleus as many neutrons as to stabilize such a large number of protons.
Radioactive response
Radioactivity is the process of balancing leftover or missing neutrons. In both cases the process is very different. Particles and energy are emitted in both, but very differently.

The most abundant isotopes in the universe are 1 hydrogen and 4 helium.
ANDÉN
When there are too many neutrons, the nucleus presents a difficult situation. The logical thing would be that neutrons were expelled from the nucleus, but it is almost impossible to expel a neutron without more, much energy is needed. Instead, the neutron disintegrates. Being a neutral particle, disintegration produces a positive and negative particle, a proton and an electron. (In addition, another particle and energy is released in this process.) The proton stays in the nucleus, so the atom becomes another atom by having one more proton; the isotope of carbon 14 (6 protons, 8 neutrons), for example, becomes 14 nitrogen (7 protons, 7 neutrons) by such a process. On the contrary, the electron is ejected with great energy. This radiation is called beta.
When neutrons are too low, the core uses another strategy to compensate for this lack: it emits alpha particles. Alpha particles consist of 2 protons and 2 neutrons, that is, they are helium nuclei 4. As already mentioned, they are very stable, so it does not take much energy to eject these units from the core. The nucleus loses two protons (it becomes a smaller atom) and thus somehow alleviates the need for neutrons. It doesn't need so much neutrons to stay stable. For example, the famous uranium-238 is transformed into thorium-234 by emitting an alpha particle.
Radioactivity of waste
Bismuth 209 minor atoms hardly emit alpha particles, usually heavy core radiation. In fact, elements related to waste issues in nuclear power plants emit alpha particles. An important example is the radio-226 isotope, a product of uranium fission. In this type of isotope, if radiation were treated, alpha particle emission should be treated.

Isotope stability depends on the proportion between the number of protons and neutrons.
Archive
We should accelerate. Thus, instead of emitting radiation for many years, it would run out in the shortest possible time. The isotope Radio-226 itself has an average life of 1,600 years. This means that at that time half of the amount of radio is disintegrated, and at the same time half of that half is disintegrated, that is, a quarter of the initial amount remains.
Think about how long to wait until the entire radio disintegrates. Therefore, the underground underground is not a good solution, as the problem persists 'forever', even if it is underground. And that's why physicists want to invent a process that accelerates this radioactivity. Instead of burying the problem would disintegrate. It is believed.
The world of electrons

The atoms of metals are arranged in mesh. In this network the cores are standing and some electrons move freely throughout the network. Therefore, the network is a medium with many electrons and can be used to accelerate the emission of alpha particles.
Archive
Several proposals have been made to accelerate the emission of Alpha particles. Environments with many electrons have generated the most hope. The idea is simple: alpha particles have a positive charge because they have two protons (as the name suggests, neutrons are electrically neutral); if they are immersed in a medium with many electrons, the medium would "pull" alpha particles out of the core because electrons are negative charges. Therefore, this negative medium would accelerate the emission of alpha particles.
It is, of course, about finding a suitable environment. The last proposal was made by physicists from the Ruhr University of Germany. The Polonio-210 isotope is trapped inside a metal and its low temperature. The atomic network of metal is a medium full of electrons that cools so that atoms remain as slow as possible. In this way they have affected the radioactive isotope.
According to physicists, the results are positive. Now they want to try to do the same with the radio-226 isotope. This isotope has an average life of 1,600 years and, according to German physicists, can descend to about 100 years. And with more research, a shorter half-life can be achieved.
But not all physicists believe that. In the tests carried out by physicists of the University of Oxford they have not managed to shorten the average life. In addition, in short, this methodology should be adapted to nuclear power plants. If the isotope is forcefully refrigerated, it is difficult to start an efficient process, since cooling also requires a lot of energy.

If there were a reduction in the half-life of several isotopes occurring in nuclear fission, we would be on track to solve the waste problem.
Archive
The German proposal has generated a great debate between researchers and physics blogs. In short, radioactivity control could be a utopia.
The paradox of the perfect atom
It is not practical to express the weight of atoms in grams. They are too small for it. Therefore, physicists invented atomic unity and gave the weight of all atoms according to it.
To define the new unit they needed a reference and used a perfect atom, carbon. Carbon 12 is the basis of life and the most abundant isotope, is formed by a perfect structure, with six protons and six neutrons in the core. That is why scientists decided that the weight of the perfect nucleus of the perfect atom would be 12 atomic units. Being formed by 12 particles, each of them would weigh a unit on average.
But this decision was a surprise to physicists. From this reference, they calculated the weight of a free proton and a free neutron in atomic units. The results of both measurements were 1,00734 and 1,00867 respectively. Therefore, if the measurements had been done correctly (and they did well), these twelve particles weighed more when they were loose (12,09606) than when they were together in the nucleus of the atom (by definition, 12,0000). Where was the lost mass? Einstein's answer.

Albert Einstein.
(Photo: And. Karsh)
The union of six protons and six neutrons in a nucleus requires a fusion reaction in which great energy is released. How much? According to the Special Theory of Relativity, which provides the formula E = mc 2. The more mass is lost, the less energy the core has and the more stable it is.
If this calculation is done with all atoms, it is clear that each one loses a quantity of mass by forming the nucleus, that is, some nuclei are more stable than others. (The exception is the hydrogen-1 nucleus, formed by a single proton (free), so the nucleus and the free proton weigh the same).
The most stable core is iron 56. And from there, if we ascend or descend through the periodic table, there is no nucleus that loses so much mass. Therefore, the fusion of stars generates much more iron in the periodic table than in the one around them. The exceptions are 1 hydrogen and 4 helium, since in practice they are raw materials of the fusion of stars.
Magic numbers
In the nucleus only some proportions between the number of protons and neutrons are stable. Most are radioactive. The reason for this lies in the structure of the nucleus, somehow organized by layers, just as electrons are found in orbitals. In fact, physicists found that certain amounts of particles stabilized the layers by leaving them full, reducing energy levels to the core. These numbers, called magic numbers, are:
2, 8, 20, 28, 50, 82, 126
The presence of two protons or two neutrons in the first layer stabilizes the nucleus (or two protons and two neutrons in the case of the helium-4 isotope) the presence of eight in the second. And so also with the following. This does not mean that no combination is possible, but when the number of neutrons or protons is a magic number, the core is especially stable.
Roa Bridge, Guillermo
Services
224
2006
Services
035
Physics
Article
Services












