COVID-19: Vaccines do not cause any worrying side effects during the vaccination campaign
Since the start of vaccination campaigns against COVID-19, all States have been closely monitoring to identify and evaluate any adverse reactions that have not been observed in clinical vaccine analyses. Although there is no direct data available from the Basque Country, the Spanish Agency for Medicines and Health Products has published a report of all the side effects identified from the beginning of the vaccination campaign against covid-19 to day 17. During these days 494,799 people have been incorporated in Spain, of which 374 have suffered side effects. That is, 0.08% of the vaccinations. Most side effects have been mild and without severity.
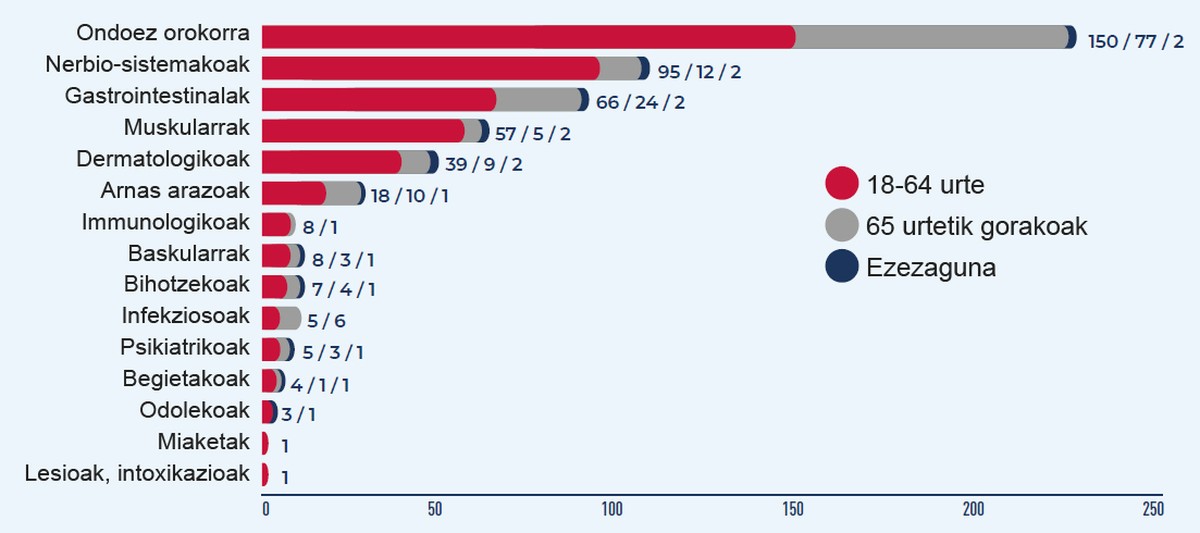
Although the Pfizer-BioNTech and Moderna vaccines are authorized in the European Union, Pfizer-BioNTech is the only one that has been issued in Spain during the drafting of the report. 70% of vaccinated people were women and as for age, 38% were older than 65 years (the other 62% were older: From 18 to 64 years).
Most of the reported side effects have been fever or general discomfort; to a lesser extent, nervous system (mainly headaches and dizziness) and gastrointestinal (nausea and diarrhea) problems. Proportionally, women have made a double communication with respect to men.
Data on allergic reactions
The report focuses on allergic reactions, since in clinical analyses of mRNA vaccines, few cases of anaphylaxis or severe allergic reaction were identified. The U.S. Center for Disease Control and Prevention identified an anaphylaxis rate of 11.1 per million doses, with 4 cases in Spain. That is, a rate of 8.1 per million doses. Of these four cases, three had a history of allergic reactions, all of them women.
Anaphylaxis mainly affects the skin, circulatory system and respiratory system. The main symptoms are urticaria, itching, hypotension, tachycardia, loss of consciousness and respiratory difficulty. It appears between 15 and 30 minutes after vaccination and, because it is serious, all vaccination services are prepared for immediate treatment with adrenaline. The four cases exposed have reacted correctly to treatment.
Since there are few cases raised, there is no reason for concern. Yes, vaccinates who have suffered an anaphylaxis reaction have been recommended not to take a second dose.





