Big Mysteries of Small Cells And How Fluorescent Indicators Have Become Information
The latest generation discoveries have turned the world around and a half. On the one hand, space-oriented research has taken us outside the earth and other planets. The idea that other forms of life have existed or exist in the Universe is stronger than ever. On the other hand, he has deepened the work on the structure of the internal particles of the atom of travel to the interior, which have also contributed new intentions about the universe. However, there are still more outstanding questions than answers.
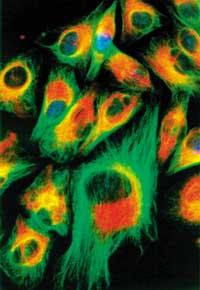
The living cell is undoubtedly the most complex and surprising structure of our planet. On the one hand, for its rich internal information and compact structure (think, for example, that the development program for the creation of a whale is stored in few nanograms of DNA). On the other hand, each cell performs a complex job in a very small place. At all times, thousands of different molecules are carrying out numerous biochemical reactions within a specific and catramical metabolic plan. At the same time, they maintain the cellular structure and also correctly perform their function in a universe of cells throughout the organism (the number of cells in our blood is higher than that of the world population). Given this demonstration, we can think that the most spectacular machine made by man is also a clumsy toy.
Most of the phenomena that occur within the cell are for the time being mysteries. However, some progress has been made and the path to research is open.
Until a few years ago, reactions were analyzed individually or collectively in glass tubes after the dissolution of cells. This strategy has made great strides. Among other things, the structure and origin, origin and destination of many cellular compounds have been known. This has made it possible to clarify the main metabolic processes such as glycolisis, the Krebs cycle, etc. However, this research, carried out outside the cellular architecture, has darkened many other details such as the actual speed, location and spatial direction of the processes that occur inside the cell. For example, the relationships between neurons inside the brain have not been obtained accurate data, since in a very small space many and complex reactions occur.
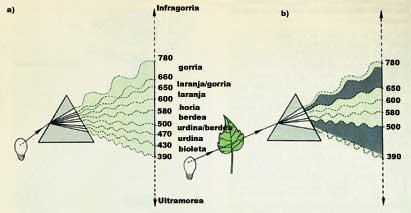
In order to overcome the obstacles mentioned above, scientists have tried to develop new techniques to analyze these life processes while maintaining cell integrity. These new techniques are based on spectroscopy. That is, once a complete cell has radiated by electromagnetic waves, the waves received through the cell or by reflection store information about the cellular structure. This is the technique used in x-rays. X-rays that obstruct the bones do not reach the film, which allows us to obtain information about the shape of the bone.
The molecular architecture within the cell is very complex, but it is composed of few types of molecules. For example, all proteins in a bacterium (about 3,000) are composed of a combination of about 20 different amino acids. Discover the fascinating ability of life. The same goes for lipids and carbohydrates. The mystery is in combination!
Therefore, and unfortunately before electromagnetic waves, all proteins are similar, so it is not possible to analyze the individual work of each of them. This is not the only problem: on the one hand, the intensity of the radiation used and the wavelength (energy) must be within limits that do not affect the cell. Moreover, the cells are largely formed by water and many types of waves cannot be used because they interfere with water.
If cell research has had to deal with many problems, something similar has happened when it comes to investigating outer space, in many cases probes have been used. In other words, the right machines have been made and subsequently sent to the space to measure the parameter to be investigated. The measurement signal is received by radio on Earth.
In the internal work of the cells, even in the face of the problems mentioned above, indicator molecules (or probes) have been created. The molecular probe should be able to perform the same work as the space probe within the cell. Basically the main characteristics of this type of probes are:
- The spectroscopic signal (response to the waves) of all the molecules present inside the cell is totally different and totally separate.
- It must be a concrete indicator of a reaction or molecule so that the researcher can investigate the phenomena individually.
- Travel inside the cell should be done without causing cell fatigue. In addition, the probe must have a specific location within the cell and known: nucleus, cytoplasm, mitochondria, etc.
- The internal structure and function of the cell must be normal at the time of measurement. Obviously, the probe that brings together all
these characteristics is not easy to create; all the characteristics of the machines we send into space must be incorporated into a molecule. Fortunately, the collaboration between organic and biochemical chemicals has also given us spectacular results in this field. Fluorescence has been your most useful tool.
What is fluorescence?
It is a curious phenomenon that some molecules explain and that has proved very useful. Since light is formed by waves, all molecules absorb the light of a certain wavelength, traverse the rest of waves or reflect back. As we know, chlorophyll is green; if we take as an example, we will see that radiating with white light absorbs blue light (which has a wavelength between 390 and 500 nm) and red light (which has a wavelength between 650 and 780 nm) and that the others are reflected back. The color of the reflected light is the difference between white and absorbed radiated light: green light (between 500 and 650 nm). Therefore, all things that have color remove something from the white light. Whites nothing. Blacks all. (See figure 1).
What happens to absorbed light waves? The waves of light, like any wave, transmit energy and the molecules that absorb the waves pass to the state excited by the energy received. To return to its normal energy state, it emits this energy in another way. In many cases this emitted energy is the heat formed by low-energy waves. In special cases, however, the absorbed excitation energy is emitted through another visible wave and the emitted light energy is always lower than the absorbed light. In other words, the excited molecule expels light from another color. And that is precisely fluorescence.
Black lights commonly used in nightclubs eject high-energy waves that we cannot see. However, these waves have the ability to excite several molecules. These excited molecules emit, in turn, a blue-whitish light. For example, white shirts, teeth and tonics appear as if they had their own light (due to fluorescence effect).
Natural and artificial fluorescent compounds are known and can provide accurate information about processes occurring within the cell when used as a probe.
Fluorescent indicators have been used to measure the internal pH of the cell. Researchers have placed SNARF and fluorescent, groups that bind protons (H+), into a fluorescent molecule. As the molecule fills with protons, its fluorescence characteristics change and this phenomenon is measurable.
Moreover, the filling of protons depends on their concentration. Therefore, as can be seen in the figure, a change in the concentration of protons implies the modification of the fluorescence characteristics of the indicator, which can also be measured with great precision.
This expert system had to introduce this type of special fluorophores to measure the internal pH of the cell without causing cell fatigue. However, the system cannot get to work in any way. There are some technical problems to overcome and not of any kind. Groups that bind protons have electrical charge and interact with water (are therefore hydrophiles).
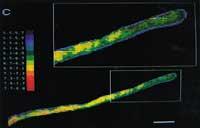
On the other hand, the cell has a cell membrane formed by proteins and lipids on the outside edge, which controls well the molecular traffic between the outside and the inside. This molecular goalkeeper does not allow you to enter any charged molecule that you do not know and because it is an artificial fluorescent indicator, it does not find entrance door. (See 2. image)
Faced with this problem, scientists have managed to disguise the charged groups by sterilization. This chemical change allows you to move from a hydrophilic molecule to a hydrophobic, which allows you to easily cross the lipid barrier in front without going through the special door. Once inside, due to the enzyme esterase containing cells, the hydrophobic ester recovers the original structure and the active fluoroforum can act as an indicator within the cell.

In some cases, the fluorescent indicator remains in the cytosol, but in others it accumulates in special parts of the internal structure of the cell. A single change in the chemical group can have great influence on the cell site of the molecule. For example, fluorescein remains in the cytoplasm, but carboxyfluoresq accumulates especially in the cowboy of plant cells. These differences have been used for special studies of cell fragments (see Figure 3).
The pH measurements of the cell have yielded very important results, but the work that has given the most fame to fluorescent indicators is undoubtedly the measurement of the concentration of calcium(II) within the cell. The investigations carried out in recent years have shown that this simple cation has a great responsibility in the internal behavior of the cell, being its task that of the second messenger.
Normally, the concentration of calcium(II) of the cell (in the language of scientists [Ca 2+ ]]) is very low in the cytoplasm (around 10-7 M) because most of the Ca 2+ cell is concentrated in special compartments. It accumulates mainly in animals in endoplasmic reticulum and plants in vacuoles.
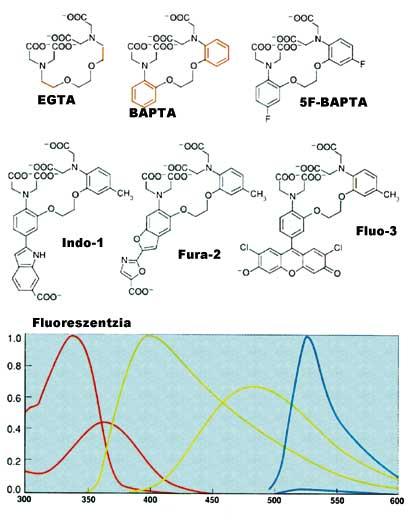
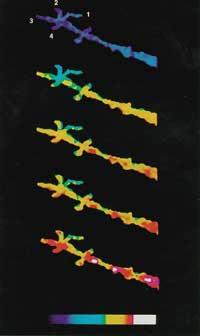
After receiving certain external signals or stimuli, the calcium stored in the compartments is expelled and the cytoplasm rises to 10 -5 M. This increase has a great influence on special proteins and generates reaction chains. At the end of these reaction chains is the expression of genes. It is therefore very important to be able to measure calcium(II) changes within the cell. The first fluorescent indicator to measure Ca 2+ was created by Roger Tsien. EGTA, from the well-known calcium complex, creates the new BAPTA structure. This change modifies the spectral characteristics of BAPTA. Unfortunately, the absorption spectrum of BAPTA is close to the rest of the compounds in the cell, leading to measurement problems. Thus, Tsien placed other fluorescent groups maintaining the molecular character and characteristics of BAPTA, forming Quin-2, Fura-2, Indo-1 and finally Fluo-3 (see figure 4).
Currently it can be measured at full speed [Ca 2+ ] of the cell. These techniques have shown that chemical responses in cells are very fast and accurate (see figure 5).
The Last Revolution: Seeing Proteins
Proteins are one of the most representative actors of cells. The information encoded in genes is expressed in proteins and these are the working molecules that make the differences. Therefore, seeing the different proteins working within the cell has been one of the main objectives that scientists have followed without getting tired. Thanks to the collaboration between genetic engineering and biochemistry, spectacular results have been published in recent years.

Within the information code that each protein has you can find the signal of your address. This signal is at the front end of the protein and opens all doors to the place you are going to go. Like the letter sent by mail, the cell would conduct a complex process of distributing each protein. Finally, once the protein is reached at its site, the direction signal is removed and in some cases separated from other functional areas. Sometimes it is still part of the protein.
Some marine animals contain proteins that themselves are fluorescent. Because of these special proteins, jellyfish have long been known and have been used as fluorescent indicators to clarify the direction of other proteins.
The bases of this spectacular technique are simple: you can isolate previously the gene of the protein that you want to investigate and place the fluorescent indicator gene behind or in the center of the gene; then a new copy is inserted inside the cell to continue its path. In the absence of artifacts, the fluorescent indicator allows to obtain an accurate and spectacular image of when, where and how much the protein investigated.
In view of existing innovations, it can be said that with the use of live cells many discoveries will be published in the future; the beauty of the results obtained will bring us much more than the pleasure of knowledge.





