Water: curiosities of a special liquid
Elhuyar Fundazioa
Molecule that does not comply with the law

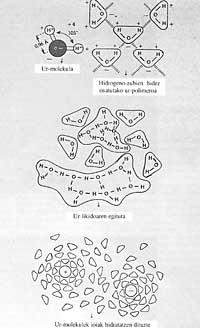
Water is a combination of an oxygen atom with two hydrogen: H 2 O, just that. A very simple and well-known formula, undoubtedly the first we have learned. Being such a simple molecule, it seems that if you want to know something about it, it would be enough to take any basic textbook about chemistry and draw conclusions from it. Its structure does not suggest other particularities.
The first thing that has always been said about water is that it is a liquid in pure state, without color, no smell, no taste. But from there the properties are very special and thanks to that particularity the life in our planet.
When Celsius invented the thermometer, it was based on two properties: boiling water temperature and freezing temperature. The first of them coincided with 100°C and the second with 0°C. He divided the space into one hundred equal parts, each with a degree. Thus was born the first instrument to measure temperature. Celsius did not know, however, that these two properties are not normal in water in terms of magnitude. Scientists know that the boiling point of a liquid has to do with the size of the molecule. That is, the smaller the molecules, the smaller the boiling point.
This means that water, compared with other molecules of its size, has a boiling temperature much higher than it would be.
This point can be analyzed from another point and the conclusion is similar. It is known that the properties of the compounds derived from elements of the same group of the Periodic Table are not of any type, but can be predictable depending on their position in the Table.
Oxygen belongs to the same group as sulfur, selenium, curtain and polonium and water, the hydride of oxygen, has the same formula as the hydrides of the elements of this group: H 2 S, H 2 Se, H 2 Te, H 2 Po. The boiling temperature of all these compounds is known and is increasing regularly from the hydride of the sufera to the polonium, which is heavier.
However, the water has not ignored this regularity and has a boiling temperature much higher than the corresponding one. Thanks to this, the water is liquid at normal temperatures and not gas, like the rest of the hydrides of this family.
Something similar happens with the freezing temperature, which is higher than that of the hydrides of other elements of the group, which should be about -100°C is 0°C.
The tortuosity of water against the laws of the periodic system makes the liquid and solid state that presents water in the soil abnormal, since according to the laws should be in a gaseous state.

Another non-normality of water is that ice is lighter than water. That is why the iceberg peaks are out of the year. Physical experience on this point is quite the opposite, since all other substances are heavier in solid state (have higher density) than in liquid state.
Therefore, if a solid piece of metal is deposited in a cast metal, it will never float, it will sink.
Changes in water density with temperature are also spectacular. All substances are dilated with heat and contract with cold. But the water does not conform only with it and has its maximum density at 4º C. If it continues to cool below this temperature, contrary to what you would expect, its volume increases until it freezes. Therefore the bottles are broken when we leave them forgotten in the refrigerator.
The glass bottle has a normal behavior, so it is contracting as it is cooled, but the water must always do the opposite and when freezing increases its volume. And if we don't go fast, the conclusion is known: the need to put pieces of glass in the refrigerator and a new bottle refreshing.

Another ecological and much more important consequence of this maximum density is the deep surface freezing of rivers, seas and lakes. Thus, the surface layer of ice acts as an insulator and can subsist under icy waters.
When, after the rain, the water that has remained in small rocky fringes is frozen, due to the enormous pressure generated by this increase in the volume of the ice, a rock break or at least the formation of cracks can occur.
Another important feature of water is its high calorific power, which also has great ecological consequences, such as the time regulator.
When the winter sends us its first cold times, the water takes ice and snow, releasing the great amount of heat it had stored. Thus, the cold winter days do not feel them abrupt, since the ambient temperature cools down more slowly.
On the other hand, when melting ice and snow, they get a lot of heat from the environment and hot days feel them more slowly.
On the other hand, the presence of hot marine currents (such as Gulf Stream) also has great importance in time regulation. This current reaches from the lands of tropical time to the cold regions of the Arctic and the Antarctic, where heat is released. The amount of heat flowing into this problem is really incredible. Let's look at the figures:

The amount of energy released by Gulf Stream on air time is equal to that generated by rivers of 200 billion tons of coal. This amount of tons is approximately 2/3 of the annual coal production. In other words, the high calorific power of water allows the oceans to absorb solar energy and turn it into a huge energy deposit. As we have said, these gigantic masses of water slowly move towards cold lands freeing warm energy and tempering time to avoid drastic changes.
It is true that we have become accustomed to a thousand things that occur daily in nature and have lost the ability to surprise us before them and curiosity to find their reason. Can you ever think why there is rain formed by almost round drops? This is due to a strange property of water.
With the exception of some liquid metals, such as mercury, and some molten salts, the surface tension of water is higher than that of any other substance. The surface tension gives the measure of the force that causes the water to not expand on a surface and to remain in the form of a drop, the same happens in oil and gasoline.
If the surface tension of the water were lower, even with the lowest wind, the marine waters would wet the coastal lands. Similarly, our rainfall and gabardinas would do little if the water had little surface tension, as it would pass through the smaller section. However, the few so-called surfactants easily reduce the surface tension of water. Because of this property that does not have the other liquids, water (sweat) can rise by about 80 m or more in very narrow capillary tubes of trees, located on top to feed the leaves.
The use of lavender soap also has its reason, the soap is tensoactive and if we did not use it, due to the high surface tension of the water, the water would not reach all the grooves, that is, it would not wet our hands well.
Water in life processes
In light of all these properties we have studied, we can say that water is not a very ordinary liquid. And we can also ensure that it is difficult to find a single property of water that can be considered normal. This peculiar behavior has great importance in many fields of science and technology, but now we will overlook the role of water in the subsistence of life on our planet.
Water participates in almost all biochemical reactions. Let's see the paths of the human body for energy. We all know, among other things, that we feed on carbohydrates, which are the energy source necessary for all functions of the body. The reaction of carbohydrates in the transformation of energy is that the burning of glucose, by oxygen, produces water and carbon dioxide.
The daily amount of water the human body needs is approximately 2.5 l. This water is obtained through food and drink, but also through the aforementioned reaction. The amount of water generated per person per day by this last route is 0.3 l. In this way, if the carbohydrates and the water consumed by people and animals are not renewed, they will be exhausted soon. The natural balance is achieved through plants, which react equally but in the opposite direction with solar energy. That is, plants synthesize carbohydrates from water and carbon oxide (IV). The plant kingdom of this simple operation requires 3 billion liters of water a day.
The reaction being studied requires 185 l of oxygen per person per day. Since oxygen in the air is only 21% and the efficiency of the human lungs is 14%, we breathe daily 6300 l of air. These figures are not related to our topic (water), but it seems convenient to refer to our environment and especially to our dependence on the work of plants.
Another aspect of the involvement of water in the processes of life is linked to its solvent capacity. It is the means of transport of substances through living organisms. To do this, water must break the bonds between the atoms or molecules of the body in solid state, separating them from the body from such atoms or molecules to remain within the water. Because of this capacity, sugar can enjoy coffee with milk or cooking salt to salt the food.
Another consequence of the solvent capacity of water is that it can act as a biochemical basis. We have just seen the water capacity as a solvent to disassemble the structure of the bodies and are now saying something that seems contradictory; it has the role of biochemical base. Although it seems a lie, there are no contradictions, because they are aspects of the same function. As for the first aspect, water keeps in dissolution simple substances such as sugar or salt, but in the second, complex substances such as enzymes help maintain the structure.
The enzymes are substances produced by nature to be able to conduct the biochemical reactions necessary to carry out the functions of the body. Enzymes are proteins, giant molecules formed by amino acids. Enzymes, like pearl necklaces, can adopt many forms (spatial). In the case of enzymes, this spatial arrangement is of utmost importance, since to fulfill its function it has to be a spatial arrangement very precise and different from all other enzymes.
Scientists still do not know well the forces that make these provisions possible, but it is certain that for this function water is necessary. If, for any reason, the composition of the aqueous solution surrounding the enzyme is modified, it loses its own form and, therefore, its capacity to perform its function.
It is not yet clear how water maintains the protein structure acting as a biochemical basis. What can be said is that water-protein junctions are very weak and are very easy to perurbar.
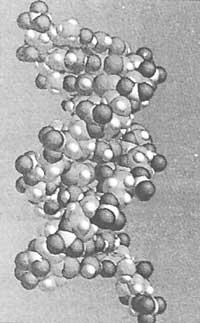
The role of the biochemical base is not limited to the scope of enzymes. The same applies to other basic biological structures such as the double helix of DNA.
In light of the two aspects we have seen, water as solvent and biochemical base, one can think that the role of water in biology is static. But, of course, this is not the case: water, as a biological fluid, carries the substances necessary for life (food, oxygen) to the necessary places and at the same time expels the residues generated by the metabolism.
To see this behavior we will give some figures. Each heartbeat pumps 70 ml of blood. Taking into account that it performs 70 pulses per minute, we have carried out simple operations and have achieved that 7000 l of blood circulates through our body. Other organs, such as the kidney, should also treat large amounts of water.

We have seen that the presence of water in living beings is important, but not all living beings have the same proportion of water. All forms of life and all tissues of a certain individual have water, but in different proportions. The 60% of the weight of the human body is water and 99.5% of the jellyfish. In our body, the brain and muscles are those that have a higher proportion of water and bones than less. It is known that each cell needs a minimum amount of water for its operation, but not much is known about the properties of water in the cell.
Its status, distribution, functions and obligations and its control mechanisms for output and input remain unknown. Therefore we do not know the cause of problems like the following. The problem is that when tissues freeze, 20% of their water never freezes, even at very low temperatures. It seems that there are water molecules that are protected and hidden and cannot occupy a place that would correspond to them in the ice crystal. This water not only appears in living beings, but also occurs something similar in microscopic pores of minerals.
And to finish, let's see how some animals and aquatic plants adapt to live inside.
The density of water compared to air is so high that any body in water is 800 times lighter than in air. As a result, the plants and animals that inhabit the water do not need a skeleton as complex as those that inhabit the soil. But not all are advantages. The insolubility of oxygen in water is limited, so people living in the aquatic environment have breathing difficulties and have had to develop complex respiratory systems.
It is evident, therefore, that living immersed in water has its problems, but it is not minor those who live in territories with water scarcity. The living beings who have adapted themselves to live in these media do not therefore have a lower dependence on water. On the contrary, the price they have had to pay has been very expensive, since they have had to develop very complex mechanisms to conserve water.

Most of the water on our planet is not sweet, but salty, with only 0.3% of the fresh water available. Most of this water is found at the Poles and the other main source of fresh water is atmospheric water vapor. The amount of water that is evaporated annually is estimated at 450,000 billion liters. To realize the magnitude of this quantity, think that if that water was uniformly expanded on our planet it would generate a water layer of 106 cm in height. 75% of this steam returns to the oceans in the form of rain and part of the rest does so also through rivers.
The amount of water in the atmosphere is only 12000 billion liters. Therefore, we will find that by making a simple division, the atmosphere water is recycled 37 times a year.
It must not be said, but we are going to say, that as the population increases and the consumption of water per inhabitant, and the proportion of the water arrows of this planet, which is useful to us, is not only a scientific problem, but also political and economic.






