Taxol: example of two faces of nature
To say that the bases of death and life sometimes have the same source is not always an occurrence. In the case that occupies us, the first ones materialize in the tree called tejo, taxus baccata, which appears in the shield of Gipuzkoa, and the second in the substance called taxol (figure 1) that can be obtained from the same tree. They are two totally different faces of the same reality.

In our mountains there are few specimens of the mill, most of them decorated in gardens. To grow, this tree chooses cold and mountainous shadows, with a height of twenty meters and a life capacity of more than a thousand years. In his youth, he has a reddish skin, but then becomes darker. Leaves, dotted and green, forming rows. As in the case of other dioecious plants, we find male and female trees, whose fruits are surrounded by a bright red aryl. The wood, without resin and very hard, both in carpentry and in carpentry, has always been appreciated, and that estimate would be the main reason that in Euskal Herria a few teeth remained.
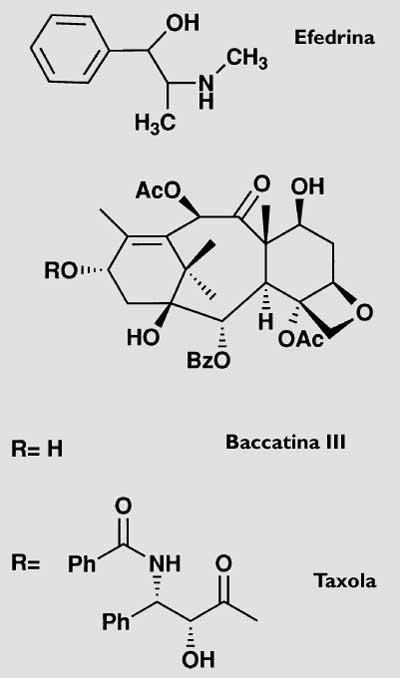
While in the aryl surrounding the fruits there is a narcotic substance called ephedrine (Figure 1), in the rest of the tree there are different amounts of a venomous alkaloid mixture called taxin. Ingestion of the latter can kill by stopping the lung. These two chemical substances are responsible for the mythology that is generated around this tree. The mythological part of the promontory, the taxus, begins to sound in its taxonomic name. From the word taxos or taxis (row), by the arrangement of the leaves, has taken the name. The yew, for its poison, gave name to the toxic substances that were initially to be taxed. It must be said that in Italy it is known as “the tree of death”. In the accounts known for the wars between Romans and Basques, the yew also appears.
They say that the Basques rubbed the tips of their arrows with poison of the muela in the attacks against the Romans. They also drank poison before falling into the hands of the Romans, killing each other. Or when the Romans put them on the cross, they ate the Arilos and overcome the pain, singing while they died and insulting the Romans, who looked at them with fear and open mouth.
Both the shepherds and the baserritarras have tried to keep this tree as far as possible so that the animals do not eat the leaves and the fruits of the muela. As we see, this tree has been related to death until the 1960s. At the beginning of this decade, the American Institute for the Fight against Cancer discovered the presence of substances extracted from different types of plants: The raw extract extracted from the bark, Hagina del Pacífico, Taxus brevifolia, showed activity against cancer cells and certain tumors responsible for the development of leukemia. Consequently, in 1971, Wall and his team of researchers isolated from the aforementioned raw compound an active compound taxol that, with the help of an X-ray analysis, completely consolidated its chemical structure.
The investigations carried out to date have revealed to us how special the taxi is. Between 1960 and 1981, 100,000 substances of 35,000 plants have been analysed, with taxol being the most active of all those found. Cytotoxic compounds stop cell repetition. Given that one of the main characteristics of cancer is uncontrolled cell proliferation, it is considered a very adequate way to combat this disease.
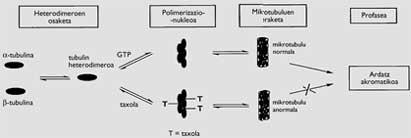
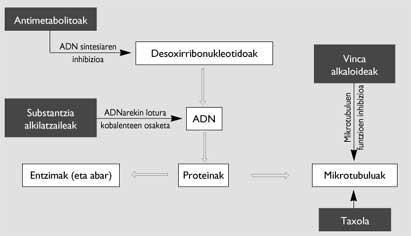
There are several compounds that present this characteristic, with different types of effects, moments and forms (Figure 2). Optionally, the alkaloids obtained from the plants of Vinca and the taxol are those that act on the microtubules. They are formed by chains of heterodimers formed by the microtubule and tubulin, formed by the polymerization carried out with the help of guanosin-5’-triphosphate (GTP). One of its functions is to form the acromatic axis necessary to channel the distribution of duplicate chromosomes in mitosis, so that the two cells generated from a cell have the same DNA.
Vinca Vinca
While the alkaloids inhibit the polymerization of the heterodimers, taxol results in a polymerization without GTP support, resulting in microtubules forming groups of non-normal chains. Therefore, the achromatic axis is not completed properly and the mitosis of cancer cells remains in the passage called profase
This mechanism, which has made the taxi such an interesting place, allows to achieve a very solid cytotoxic activity against cancers of leukemia, ovaries, lung, breast and melanoma. According to trials conducted in recent years with hundreds of patients, many cancers are cured. But a taxol with such a miraculous effect against cancer is not yet on the drug market. The reason for this is explained by two reasons: on the one hand, for the existence of problems to obtain this substance. For example, to obtain a kilogram of taxol it would be necessary to cut 3,000 trees and then perform very complex extractions. On the other hand, being a hydrophobic taxi the direct ingestion is not adequate, hypersensitization reactions are produced by auxiliary substances used as a driver. The need to resolve this last problem has delayed clinical trials for five years.
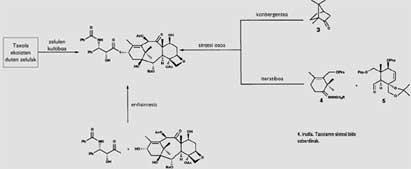
The direct obtaining of the taxi from the trunk of the muela is an enormous ecological damage. But fortunately there is a way to address this problem: chemical synthesis. The synthesis can provide the necessary taxi to heal people and avoid the yew. Therefore, this way solves the two problems at a time. Both the chemists and the biochemists, nothing more to know the structure of the taxi, began their synthesis. Figure 4 summarizes the different routes followed in this gigantic work. First, it is worth mentioning the synthesis obtained through cell cultures. The key to this path is finding cells that synthesize taxol. These are grown in vitro, collecting the taxol they produce. Several industries working on this path have obtained very good results when cultivating cells extracted from the taxus brevifolia.
However, the most profitable way to date is that of the semisynthesis, that is, the direct extraction of part of the molecule in the plants and the obtaining of the other part through chemical synthesis. The two parts that should be joined in our case would be a derivative of the muela, taxus baccata, baccatina that is extracted directly from leaves III (Figure 1) and phenysoserine that can be synthesized chemically. In this case, in addition to being a simpler extraction than that of the taxol that leaves the trunk of the muela, the yield is much better (to obtain a kilogram of baccatina III it is necessary 3000 kilograms of leaves and also, as the leaves are renewed every year, it can be repeated continuously).
It should be noted that on the synthesis of phenysoserine numerous methods can be found, one of which materialized in 1990 in the Department of Organic Chemistry of the Faculty of Chemistry of San Sebastian. This work was the beginning of the most appropriate methodology currently for taxi synthesis.
It is worth mentioning, on the other hand, the work done during these years to obtain a complete chemical synthesis of the taxi. The last part of the historical length of this synthesis has arrived in 1994; R. A. A. Holton and K. C. C. Two groups of researchers, led by Americans Nicolaou, have achieved a complete synthesis of taxol following two different paths. Holton, starting from Kanford ( 3 ) and using iterative synthesis, has obtained baccatina III. However, Nicolaou, making a convergent synthesis with ( 4 ) and ( 5 ), has reached the same compound.
It should be noted that while both summaries are suitable at the laboratory level, the extension of the process to the production level still requires a great simplification of the methodology used. However, this does not diminish either Holton or Nicolaou, since this synthesis has meant a very important advance in the chemical synthesis of natural products. At this time the researchers are trying to obtain compounds similar to taxol that, in addition to maintaining or improving the activity of taxol, could overcome their limitations, such as hydrophobia indicated above.
This case shows us the uniqueness of Nature, since poison and medicine appear in the same tree. The history of Taxol shows us the complexity of nature: a single tree, a yew, an old poison and the current drug. Only the wisdom of the human being can distinguish it by its damage or by its health.
Soon the taxi will go on the market as a drug and we hope all expectations will be met.
Thank you:
Professor Jesus Mari Aizpurua and Iñaki Ganboa thank you for this article
for the ease and value they have given me to write. Regina, Txema and Mikel were incomprehensible
For making statements understandable. And finally I would like to thank Jon Andoni Arozena.
1.
all the bibliography obtained.