Penicillin, good imitation

In 1928, in a laboratory in London, he tried to grow a colony of bacteria. He tried, but he did not, and there was no food problem, as biologist Alexander Fleming fed bacteria for experimental use. However, bacteria had a growth problem.
They could not increase the outer wall as they grew. And the reason was that bacteria could not add one of the molecules that form the wall, the dimer of alanine. Instead of taking this dimer, the proteins that were building the wall received another molecule and were blocked. Therefore, the wall of bacteria did not increase at the rate of growth of the bacteria itself. And because they had an incomplete outer wall, osmosis caused bacteria to explode.
Fleming did not see everything, he did not know that bacteria could not build the wall. But he discovered that next to the bacteria colony there was a fungus of the species Penicillium notatum. All the bacteria surrounding the fungus were dead, so the fungus should be a killer. In addition, he realized that the weapon of the fungus should be a chemical, a molecule. Fleming called him penicillin.
The journey of penicillin
If a fungus used penicillin to kill bacteria, why didn't man use it? Fleming sent some of his cultures to the University of Oxford. There, Howard W. Florey and Ernst B. The Chain team began conducting clinical trials with penicillin. Very good results. Penicillin killed many types of bacteria, was a broad spectrum antibiotic.
Since then many researchers began working with penicillin, especially in the pharmaceutical industry. The objective was, on the one hand, to clarify what was the structure of the molecule --to understand the functioning of penicillin and to be able to synthesize the same molecule in the laboratory- and, on the other, the need to find a way to produce large amounts of penicillin from the fungus.

World War II began and access to penicillin became very important. According to some historians, penicillin research was more important than the Manhattan Project itself, and the antibiotic certainly saved more lives than atomic bombs.
The first patient treated with penicillin was a UK policeman in 1941. An infection in a mouth wound spread to the eyes and lungs. But the effect of the antibiotic greatly improved its condition. However, they had little penicillin and although they tried to recover the antibiotic from the patient's urine, finally the man died. Paradoxically, the treatment was successful: man could be treated with the new antibiotic.
Imitation
The secret of the influence of penicillin is imitation. The wall of bacteria is a very complex structure, formed by glucids and peptides. But just put an obstacle to a single component of these complex structures to curb the growth of the entire wall. A single component, but not just anyone. What mimics and therefore represents the penicillins is the dimer of alanine.
Penicillin closely resembles the dimer of alanine, with the same atoms (plus other additional atoms) in the same order and in the same geometric structure found in alanine. Precisely, the additional atoms contained in penicillin support others to have a straight geometric structure.
Geometry is very important. If penicillin did not have the same geometry as alanine dimer, the bacteria proteins would not take it. Bacteria cannot be deceived if their weapons have not been used. In fact, the alanine that is part of the wall of bacteria is not the same that most living beings use.
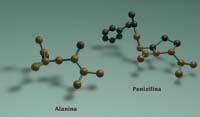
Alanine, and most amino acids, are asymmetric molecules with two main forms: the left and right forms. L-alanine and D-alanine, respectively. The problem is that in the proteins of most living beings only appears L-alanine. But bacteria use another form on the wall, D-alanine.
It is a weapon of bacteria, since other living beings cannot interrupt peptides formed by amino acids in the form of D. L-amino acid peptides are destroyed by conventional proteases. And that is the gift of penicillin: It is the imitation of d-alanine. That's why it works against bacteria.
Moreover, it works against many bacteria. In all bacteria the wall is different, some components vary from species to species, but D-alanine appears in most cases, so penicillin is a broad spectrum antibiotic.
A ring
Penicillin works, but it was not easy to figure out how it is. The researchers were motivated, World War II was underway, penicillin was scarce and much needed, and one of the hopes was to synthesize this molecule in the laboratory. What he got had good reasons to dream of the Nobel Prize.

However, at the end of World War II, it took twelve more years to synthesize penicillin in the laboratory. John C got it in 1957. Americans Sheehan, at MIT. A synthesis pathway goes through knowing first how the molecule is to be synthesized; the crystallographic work of the British Dorothy Crowfoot Hodgkin revealed the structure of penicillin.
It was the structure proposed by Florey and Chain (from the Oxford team). The base of the molecule consists of two cycles in which the secret of the success of penicillin was the smallest.
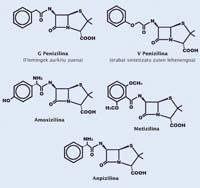
It is a cycle of four atoms, three carbon and one nitrogen. A carbon next to nitrogen has associated an oxygen by a double bond. Chemists call this combination of atoms amide and lactam amides in the form of a cycle. Those of four atoms are called beta-lactam. In fact, all penicillins (methicillin, amoxicillin, etc.) are antibiotics due to the beta-lactam cycle in the structure, cycle that gives the molecule the same geometry as alanine. Therefore, this type of antibiotics are called beta-lactam.
Bacterial response
The success of penicillin and all beta-lactam antibiotics is not always guaranteed. They are powerful weapons during intermicrobial warfare, but bacteria also have the ability to develop defense weapons against antibiotics. Fleming himself discovered that if the fungus did not kill the entire bacterial colony, over time penicillin did not affect the surviving specimens. They developed antibiotic resistance.
In fact, bacteria produce new proteins, beta-lactamase, which break down the betalactam cycle. The cycle of the four atoms is undone, the antibiotic does not work.
The fungus can do nothing against this resistance. All bacteria in the colony must die from the beginning. And man should also do it, but it has not happened. Many bacteria have developed penicillin resistance. A major problem is methicillin-resistant Staphilococcus aureus bacteria. In hospitals they know it perfectly and it is or can be a serious problem. But man is not a fungus and has developed synthetic chemistry to advance the war against bacteria.
Among other things, medicines against beta-lactam can be synthesized, such as clavulanic acid, which seems a paradox, but whose molecule has a beta-lactam cycle in its structure.
On the other hand, small chemical changes in penicillin have allowed synthesizing new antibiotics that the bacteria cannot reduce. Of course, these new molecules also have to be a good imitation of the dimer of alanine, but for this reason, chemicals have many possibilities of making new penicillins. However, over time, some bacteria will develop resistance against the new penicillin and the game will start again.
But how long? Bacteria reproduce very quickly, evolve very quickly, meaning they can develop resistance quickly. Humans have the ability to respond, but not as fast as bacteria.
Last Resort
It is sometimes key to change the strategy and use other types of antibiotics, such as those without beta-lactam. Reappearance of resistant but less rapid bacteria.
In this game antibiotic bancomycin is considered the last option for the most serious cases. Bancomycin and other antibiotics in your family also attack the wall of bacteria, but not imitating alanine. However, some bacterial strains have also developed their resistance to bancomycin. And the worst thing is that they do not know how the mechanism of certain resistances is.
The discovery of penicillin has saved many lives, but it brought man into a chemical war and it is unclear who will win that war.