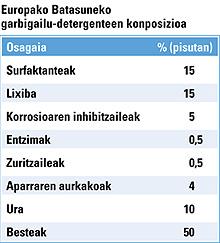
How are garments cleaned in the washing machine?
A little history
Soap was first used about 5,000 years ago. The Babylonians were the first to obtain the soap, cooked in powder with various fats, which apparently were used to decorate the hair. Since then, soaps have been present in civilizations of all time. In Egypt, for example, long ago

3,500 years, people of high level bathed assiduously with animal and vegetable fats and soaps based on alkaline salts. Also the Romans and Greeks frequently used soaps for cleanliness and medicine.
According to various legends, the Romans gave the word soap to the product that cleaned so well. It seems that on Mount Sapo the slaughtered cattle are burned and that the later generated ashes are easily mixed with the animal fats poured. I don't know how, these mixes poured into the Tiber River and came to the place where women washed their clothes. According to these women, the clothes were cleaned more easily at the top of the mountain after pouring this curious mixture into the river.
In the Middle Ages soap with olive oil was made in Spain, France and Italy. Modern soap production began in 1811, when French chemist Michel Eugene Chevreul investigated the characteristics and chemistry of fats, fatty acids and glycerin.
But the detergent is newer. It first appeared on the market in 1907 by the hand of the German company Persil. This detergent, in addition to traditional soaps, contained sodium perborate, sodium silicate and sodium carbonate (perborate + silicate = PERSIL).
The most obvious difference between detergents and soaps is its origin. Detergents are synthetic and are obtained from oil fractions. Soaps are natural. Despite being very good cleaners, the performance of soaps decreases considerably in the presence of water and mineral salts. Detergents, on the other hand, increase in the same conditions.
Cleaning mechanisms
To understand how cleaning occurs, attention should be paid to the interaction of three elements: washing, in this case the fabric; dirt or stain, that is, washing at 100 (fat in general); and aqueous dissolution in which the detergent is dissolved.
The key to cleaning is the right relationship between the three, but it has not been easy to know the details of the relationship. The behavior of detergents has been understood thanks to research carried out in recent decades, despite the centenary of its first use. In fact, as with many cosmetics, manufacturers do not know the function of many components. However, they know that the products serve and fulfill their task well.
To facilitate the explanation of the cleaning process you can divide the steps that are given in the washing machine into two parts. In the first, in the cleaning of the tissue are extracted from the tissue the drops of stains that stain the tissue. In the second, the challenge is twofold: to ensure that these drops remain dispersed in detergent solution and do not return to the fabric. It is clear that the second part is as important as the first one, since in the second, if things are not done well, the tissues will remain dirty.
Keep in mind that the cleaning mechanism is not the only one. There are many types of fabrics and dirt, and not all act the same, so it is impossible to find a single theory or mechanism that explains the cleaning process well.
If the interaction between the stain and tissue is chemical, for example, the covalent bond must be broken by additional chemical bonds between the two. Enzymes or bleaches are used for this purpose. For example, protease and amylase enzymes break the starch and protein chains of chocolate, milk, or grass stains, and completely break down the stain. But if the stain and tissue are attached by electrostatic forces or weak intermolecular attraction forces, the cleaning mechanism is totally different. It is the case of solid particles and drops of fat. In these cases surfactants are used to help stains remove them from tissue.
Surfactants, also called surfactants, are one of the most important components of detergents and have several obligations. They reduce the surface tension of the fabric and the stain and reduce the work of extracting it from the tissue; in addition, surfactants tend to be placed on the surface of the stain, so they emulsify it very easily in the water. Finally, fat drops are well protected from each other to prevent them from rejoining.
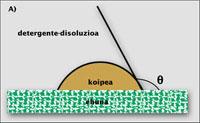
If the stain is liquid, the cleaning process can be explained mathematically in a simple way. Put a drop of oil or fat on a tissue or fiber. The drop forms an angle with the fabric, which is the contact angle (q). This angle indicates the difficulty of cleaning the tissue: if it is small, the contact surface between the stain and the tissue is elevated, which makes it difficult to clean. If the angle is high, it happens backwards. It is logical since you have to do a physical job to bring the droplet of fat from the surface of the tissue to the aqueous solution.
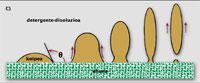
The lowest work is done when the angle q between the fabric and the droplet is 0. Then, the two touch each other at a point and the cleaning is instantaneous. Above zero, work only increases. However, if the angle is less than 90º (B), the question is not so complicated, since the help of surfactants is usually sufficient to remove the stain from the tissue. However, if the angle ranges from 90º to 180º (C), only a part of the oil drop leaves the fabric and the clothes remain dirty when leaving the washing machine. In this case, washing clothes requires another mechanism, such as dissolving oil drops.
Once cleaned, protect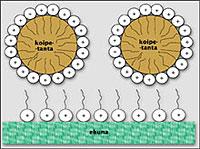
Once the droplets of tissue spots are removed, they should be kept dispersed in the water so that cleaning is not a work of discouragement. To do this, the drops of the tissue and the tissue of the drops must be protected. The collaboration of surfactants is also essential in this process.
Surfactants tend to be placed on the surfaces of drops and grease tissues, providing both surfaces with the same type of load. Consequently, the repulsion forces allow the stain drops to not match each other and stay as far away as possible from the tissue. In addition to causing electrical repulsion, surfactant molecules also physically interfere with high molecular weight chains. Surfactant molecules are also placed on the surfaces of tissue and fat droplet, and their long, curly chains help keep surfaces away from each other.
ExtrasPolymers and other products are used as additives. Sodium carboxyl cellulose, for example, once the cellulose fibers are cleaned, creates a protective layer on the surface of the fiber that prevents the replenishment of the fat drops.
Detergents contain components that do not aim to ward off the stain. Silicates and phosphates, for example, improve the performance of detergents, forming soluble complexes with Ca+2 and Mg+2 ions present in water to prevent the formation of calcareous sediments in water pipes. However, its impact on the environment is affected by the search for additives that replace phosphates in recent years.
Fluorescent dyes provide better brightness and appearance to white clothing. They absorb ultraviolet light and emit a light blue color that, thanks to this trick, hides the bitter that clothes can have.
Once done all this way, the clothes should come out clean from the washing machine and, even though there are many improvements — we all know a stain that seems to stick once to a garment and stay in it forever — it is usually so. There inside, more than a miracle, there is much at stake in chemistry.
Materials that are cleaned without soap


Some German chemists, copying the characteristics of the lotus plant leaf, have developed stones, paper or fabrics that are only cleaned. Lotus leaves are superhydrophobic (Greek hatred of water), where water drops slide completely. Even on rainy days the leaves remain completely dry. This delicacy is very interesting, since raindrops drag the dirt that lies above the leaves (dust, pollen, etc. ). ).
Botanist Wilhelm Barthlott discovered why in the early 1990s. Lotus leaves owe their acidity to the 5-10 micrometer surface itching. Pillows allow water droplets to only be in contact with the surface of the leaves at some points and cannot remain on them. They fall on their own.
Two non-natural superhydrophobic surfaces appear in the left image. In both cases, the drops that will slide easily are perfectly round. These surfaces can therefore be cleaned without soap or additive, only thanks to the movement of the drop. The effect of the lotus leaf is achieved by chemical treatment of surfaces. The method was patented by Barthlott himself with the brand Lotus Effect (BASF) and has already been used to clean facades.
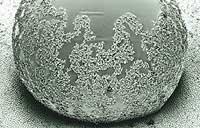
The Lotus effect allows modifying the surface characteristics of many materials and greatly facilitates cleaning. As can be seen in the image, water drops, as they move and fall, collect dust and dirt and carry them with them.
Textile softeners

Textile softeners are also of great importance in the cleaning process. They are one of the components of laundry detergents, but often fabrics are added after cleaning them. The active components of the softeners are surfactants. They stick on the surface of the tissues, placing the heads with positive charge towards the negative tissue in the water and the tails outward. They produce on the surface of textile fibers a soft layer that, in addition to protecting the fibers, gives clothing a soft consistency.
BIBLIOGRAPHY
- Kaoru Tsujii Surface Activity, Principles, Phenomena and Applications, Academic Press (1998).
- D. J. Shaw Introduction to Colloid and Surface Chemistry, 4th Ed, Worthbutter-Heinemann Ltd (1992).
- Lotus effect: Recent searches Erbil, S.L. Demirel, et al. Science 299, 1377, 2003.
Thanks to Idurre Furones for their amendments.