Billions to detect

When they observe water, human eyes see a transparent liquid. But if we look with the small eyes of the chemicals everything changes. Water is not water, but a complex mixture of millions of chemicals. Similarly, wine is not wine, a piece of cheese is not cheese and an athlete is not an athlete. They are mixtures of many molecules.
The case of water is indicated, for example, on the label of water bottles. In addition to water, the liquid contains chloride ions, sulphates, nitrates, etc., and many other substances that are not specified in the label. And sometimes the ions that are not considered in water are also represented. "Water without sodium". What does that mean? That there is no sodium ion? Normally no, it means that sodium, if it exists, is not detectable. In practice it is the same, what cannot be detected does not exist. But perhaps the problem is the technique used for analysis.
The chemicals do not detect a single molecule. They cannot. Neither two molecules; or ten; or a thousand. They must contain at least one billion molecules to detect a substance. It seems a billion, but it is a nonsense if we talk about molecules. There are many more molecules in almost anywhere.
Small and numerous
An example is an essay full of water. The water molecule is small, but very abundant. In order to visualize the water molecules, we should multiply the session by ten million times, from ten centimeters to a thousand kilometers at least. If one of the ends of a program of this size was in Bilbao, for example, the other would be in Cadiz, approximately. If the water molecules increased in the same proportion, they would have a length of two millimeters. Yes, it would be difficult to separate the molecules because they would move very quickly. But it serves to make an idea: think how many molecules of two millimeters enter an essay of a thousand kilometers in length.
The physicist Amadeo Avogadro found the quantity: 18 milliliters of water at 6,023 x 10 23 molecules, almost one quadrillion. One million has six zeros and one billion has twelve zeros, while the number of Avogadro, rounded, has twenty-three zeros. It is a very large number; if a drop of one billion molecules is removed, it seems that the change would not be noticed (in a drop of water there are approximately seventeen trillion molecules).
The truth is that the water molecule is very small, the organic molecules are much larger and yet the same happens: if a slice of ham is removed a billion molecules, the owner would not be aware of what is lost. In this sense, the detection of a billion molecules seems like a miracle.
Separation of molecules
Following the same example of water, the problem of the detection limit can be analyzed. Imagine that in this jungle of water molecules lead atoms are mixed, that is, the water is contaminated at a concentration of 50 ppm (50 grams of lead in a million grams of sample). Knowing the weights of water and lead, it can be estimated that there are about 231,100 water molecules per atom of lead. In addition, water molecules are larger than lead atoms. Therefore, the detection of lead seems almost impossible. How can you do it?
It is clear that seeing molecules is not the proper method of detection - and if there are visualization techniques -, since in this case, in addition to locating lead atoms, we should know how many water molecules correspond to each of them.
But you can also do something else: before you begin to detect, divide the molecules into groups. It depends on size, electrical load, chemical behavior, etc. Once the molecules are divided into groups, each group will have fewer molecules to identify and a more detailed detection can be made than with the initial sample.
This is what is done in the most accurate detection methods currently available: mix the sample with a liquid or a gas and insert it into a chromatograph. The chromatograph is a molecular separation tool in which the molecules have to make a path full of "trabas"; some molecules form the path quickly and others look costly. At the exit of the route, the molecules are divided into groups depending on the time they have had to complete the route. The installation of a detector in this output allows to perform analysis to each group as it comes out.
Liquid or gas
The greater the distribution, the more accurate is the subsequent analysis. It's about finding out which chromatograph will make the best separation. The doubt occurs between the chromatograph of gases and liquids. It is believed that the mixture of the sample with the gas and the gasification of the sample itself is better option than the mixture with the liquid. In short, a sample of a given volume contains less molecules if it is gas.
But, as in everything, there can be problems. Not all molecules are suitable to be in gaseous state, for example if they are very large. In addition, to transform it into gas it is necessary to heat the sample and not all the molecules overcome this process: they degrade much before evaporating. In this sense, the liquid is more useful. A solution is simple when selecting the appropriate solvent.
Therefore, depending on the substance to be detected, the distribution is done by a chromatographer or another. The same applies to detectors. Choose the most suitable depending on the substance to be detected. Some detectors are very specialized, they serve to detect a certain substance, but with others they are not accurate or do not serve.
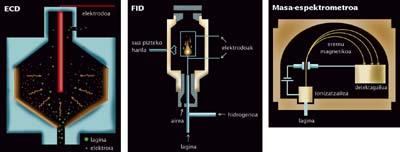
In the ranking of detection of the smallest quantities of substances, at present, three must be cited: FID, CECIand mass spectrometer. Behind these names are the most accurate detectors in the world, the best detectors that exist to search for molecular traces. The three are used in conjunction with gas chromatographs. But not always. The pair formed by the mass spectrometer and liquid chromatography is also achieving great success in recent times.

Inexact Inaccurate
Four methods offer surprising results. For different reasons it is difficult to compare the four, but if we had to choose the most eye-catching one would be the gas chromatograph/pair SICAL. The DIFF detector is an extremely accurate detector: it can detect only a few piccograms of a substance (a pycogram is one billion one gram). If, for example, we used it to detect vinyl chloride, about one billion molecules would be sufficient to detect ECDs.
Couples forming the FID and the mass spectrometer with gas chromatography are less accurate. Nanograms "only" are detected, for example, need a billion molecules to find traces of vinyl chloride.
And what success does the liquid chromatograph have? Normally, instead of giving the weight that the chromatographer can detect, vendors give concentration. The most optimistic data are surprising: some teams are able to find concentrations of 500 granulomas per liter, that is, keeping the example of vinyl chloride, would detect ten million molecules in one liter of dissolution.
User version

The numbers should be taken very carefully. These data are data from vendors who indicate the limits of detection of the method. But reality is always more complex.
Each method provides detection limits under certain conditions. And by measuring certain molecules. The CECIdetector is very good with halogenated substances (that is why vinyl chloride is a good example for its chlorine content). For its part, FID detects carbonated molecules (many are but many do not contain carbon).
However, you can also say the opposite. Current techniques meet the social need for precision in detecting substances.