Destination: molecular motors
The DNA of the virus begins to give orders when it enters a cell hostel and puts at your disposal the machinery of the cell. Therefore, viruses only need DNA to advance. Strip, DNA, and a cap or capside to protect it.
The hostelry cells, on the one hand, make several copies of this DNA of the virus and, on the other, of the catheter. DNA enters the inside of the catheter in a next step, and it is then when the new viruses are willing to leave this cell and attack others. "In half an hour of a virus, eighty viruses occur," explains Carlos Bustamante, a biophysicist at the University of California.
Specifically, Bustamante talks about the phi-29 virus, which for ten years have been researching in its laboratory. Last fall, Atom by Atom visited San Sebastian, where he exposed his research on this virus.
The Phi-29 virus is a virus that attacks bacteria, but its behavior is very similar to other viruses that affect us humans such as poliomelitis, herpes or chickenpox. Therefore, the mechanisms they observe in one and the prevention strategies of the disease can serve, probably, for others.
The engine as a destination
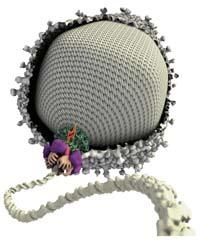
In this case, the researchers have focused on the mechanism that assemble the virus, that is, the mechanism that introduces the DNA into the cathode, in which small molecular motors, small machines on a nano scale, intervene, which give this essential step for the formation of the virus.
"If we managed to eliminate these engines, we would avoid virus formation," explains Bustamante. In this way, virus infections could be radically combated. But to reach this goal it is convenient to know to the maximum the engine.

According to Bustamante, to put the virus DNA into the catheter, "the engine has to do a great job." In fact, DNA must compact six thousand times its volume in freedom. "Too many spaghetti for such a small head," he says.
The motor has an annular shape so that from the central hollow the DNA is introduced into the hood. It is an engine formed by several groups of proteins and enzymes, among which is the core of all this work or, as Bustamante has said, "the true pistons of this engine". This is a set of five ATPases. ATPases are able to break a certain bond of ATP molecules; by breaking them, energy is released and with this energy, ATPas modify the conformation. That is, the ATPs convert the chemical energy into mechanical energy, as do the pistons of the conventional engines.
In this change of forming, the ATPases contract towards the inside of the catheter and with this movement the motor introduces it to the DNA hood.
Goliat in front of front
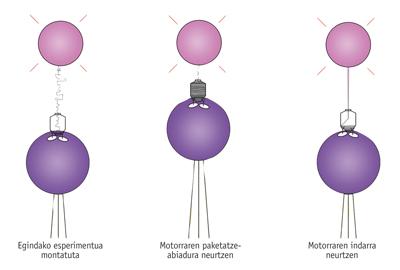
Upon learning about its operation, Bustamante and his colleagues wanted to go further and know its "technical specifications". That is, they wanted to know what strength it has, at what speed it puts the DNA inside the cathpsyde, etc. For this purpose they acted in some way in the "sokatira" with the virus, that is, they took the cathossid and the DNA by the two ends and measured the forces.
On average, it was observed that the virus introduces 100 base pairs per second in the catheter. However, speed decreases as the DNA accumulates in the cathode, due to the increasing internal pressure of the cathode.
As the speed decreases, they saw that the necessary force to perform this work is increasing. As Bustamante described, "this engine is equivalent to Goliat among molecular engines, the most powerful engine ever measured." In fact, when you have most of the DNA inside, that is, when you have to make the most of the force, the engine can become a force of the piconewton 57-60. "It's a huge force," says Bustamante, and compared it to the capacity of other molecular engines. "For example, myosine that allows the contraction of our muscles also has ATPases, but each of them can only exercise a force of five piconewtons."
With such force the DNA accumulates in the catheter to 60 atmospheres. For example, champagne is located at 5-6 pressure atmospheres in the bottles.
Of course, to make all these forces, viruses must squander a lot of energy; "if the cathode had a double size, for example, they would save a lot of energy," explains Bustamante. But "the goal of the virus is not to save energy," he says. In short, this energy that uses the engine should not be generated by the virus, is owned by the host cell, so "you do not mind wasting energy".
And also, all this is useful for the virus. The new viruses, when leaving the host cell, pass to a medium without ATP: the intercellular. There they have no access to energy, and that is when they take advantage of all the dissipated energy in the host cell: when they reach another host cell they inject the DNA they have stored at a pressure of 60 atmospheres. "This potential energy is transformed into kinetic energy and puts DNA into the new host cell under pressure," explains Bustamante. In his opinion, "it is a very elegant strategy".
Against viruses and more
Known all these details of the molecular engine, it is evident that a very effective anti-virus strategy could be achieved by eliminating molecular engines.
"But it is not enough to study the mechanics of molecular engines," explains Itziar Alkorta, a professor at the Department of Biochemistry and Molecular Biology at UPV. "Undoubtedly it is important the research they have carried out, but if you want to design a drug to eliminate this group of proteins, it is necessary to also carry out biochemical studies to know the characteristics of the proteins and to be able to choose compounds that can combat them," added Alkorta.
Precisely, Alkorta carries out biochemical studies with another molecular motor. This engine is not associated with viruses but bacteria. "We are investigating a molecular engine that participates in the conjugation of bacteria." The final objective of this group of researchers is to eliminate conjugation, among other things because resistance to antibiotics is propagated through conjugation. Conjugation is the exchange of DNA observed in bacteria, through which bacteria share a lot of information.
Once you have all the necessary information and know in detail all the aspects that should be known about molecular engines, it will be easier to develop anti-engine drugs. "The hardest thing is to know the machinery of a concrete system, that is, how it works at a molecular level," explains César Martin, another member of the same department of the UPV. Then, with this information, it is "easy" to search for ways to remove the system. Alkorta share this review: "It's not something you get from day to day, but it's not a difficult task."
But although it is not difficult, it is something to be controlled with great care. In fact, "the attempt to stop the engine of the virus could also run the risk of stopping the engines of healthy cells," said Alexander Bittner, head of the CIC nanoGUNE self-assembly group.
In addition to acting against them, the exhaustive knowledge of the mechanisms of molecular motors can allow to devise biotechnological tools on a nano scale by imitation. In short, in all cellular processes related to movement, such as the movement of flagella, cell duplication and muscle contraction, some molecular motor is involved. Therefore, we will be able to imitate any system of this type when we learn to imitate or manipulate engines, and we will be able to provide engines that make mobile to mobile cells or nano scale machines.
For example, they can be used for gene therapies. "Gene therapies basically consist of introducing a piece of DNA into certain cells to avoid a process that is occurring in the cell thanks to this DNA or, on the contrary, to promote something that is not happening." On many occasions, modified viruses are used as vectors to introduce DNA into cells. But it would be much more effective than we could only use molecular motors, as we would be able to pump DNA directly into the cells.
However, these objectives or applications are still too far away. "I like to have feet on the ground, and that is in the future; today it has not been possible to do that, that I know," says Alkorta. However, he emphasizes that it is not too little: "Before or after, we will be able to work with this type of systems. Let's go on the road."