A window to hope...
To hope they have opened a window...
A few years ago the well-known song sung by the group Oskarbi, composed of a beautiful poetry written by the Franciscan friar Bitoriano Gandiaga. And that's what we can say today about the new vaccine against malaria.
But thinking that among readers there will be more than one clutter, to tell me things from the beginning. The problem is that the malaria vaccine marketed by Colombian researcher Patarroyo has aroused great hope in the treatment of this disease (and that is why we have taken the “poetic license” for the title).
I have said a new vaccine, and we must recognize that it is not so new in itself, since in 1988 he finished many years of work at the Instituto Inmunológica de Santafé de Bogotá with a large team of researchers led by Manuel Patarroyo. The vaccine, baptized with the technical name SPf66, has great and very important news. And this for three reasons:
- Because it is the first proven vaccine that protects against the disease, whether the incidence of malaria is high or demonstrated in moderate places.
- Secondly, because it is the vaccine against the first microorganism parasite for the human being, the Plasmodium faciparum.
- And finally, because it is the first chemical synthesis vaccine.
But, along with innovation, this vaccine has generated doubts and doubts among scientists around the world. According to Professor Patarroyo, born in a country without any scientific tradition, with a completely new methodology and addressing the main health problem suffered by the Third World, he created a certain environment of suspicion among scientists who did not consider that the vaccine was effective at first.
However, clinical trials in southern Tantzania (an area where the highest incidence of malaria is recorded worldwide) have changed their minds. Although the efficiency index reached by the vaccine (31%) cannot be considered very broad, compared to conventional vaccines, the SPf66 vaccine has been a historic breakthrough in the fight against one of the diseases that generates the greatest slaughter in the world.
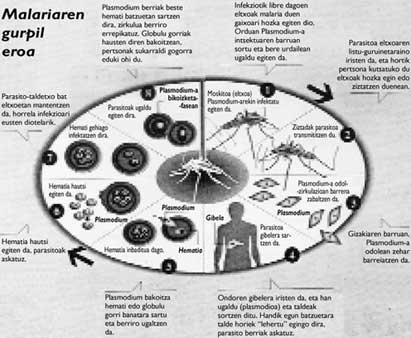
Some brushstrokes on malaria
Malaria transmission continues to take place in 103 countries (according to the latest epidemiological data) and the population of these territories exceeds 2,000 million people. Each year there are between 300 and 400 million and between 1 and 3.5 million deaths, most of them in Africa among the children of southern Sahara. Based on the results obtained in the trial, the vaccine would avoid one in three cases and, if other studies were tested, a third of the deaths.
The first trials of the vaccine were performed in different areas of South America, with an efficiency of 45-65%. However, it was necessary to confirm the effectiveness of the vaccine also in areas of greater endemicity. For this reason, they obtained the essay authorization in the population of southern Tantzania. It is estimated that in this area each person receives more than 300 infective bites a year.
In this research, randomly selected, double-blind and using a placebo-controlled control group, 586 children between 1 and 5 years old were used. Of these, 274 children received the 3 doses of the vaccine, while another 312 received placebo. This study, carried out between July 1992 and July 1993, in addition to demonstrating that after a year of follow-up the incidence of clinical diseases decreased by 31%, showed that the vaccine had a high immunogenicity and very low side effects, being the only remarkable effect in the place of injection (and only in 1-6% of cases) inflammation. It is also interesting to note that one of the children vaccinated during the investigation only died one of them, having died 5 of those who took the placebo. Therefore, it could be demonstrated that the vaccine is safe, immunogenic and partly protective in an area where the transmission of the disease is extremely high.
The immunity that is achieved with the vaccine only protects a third of children, is a failure for some scientists. But, according to physician Tore Godal, head of the WHO’s tropical disease program, “it is of great importance to have shown that protection against a parasite as fast as Plasmodium can be achieved.”
Professor Patarroyo himself has indicated that the WHO will begin in 1995 the incorporation of large groups of people in countries with the highest rate of disease transmission. And one of the points you want to know is whether the vaccine affects the mortality of the disease, an aspect that until now has hardly been investigated. In any case, a vaccine cannot be ruled out that would prevent one in every 3 people from attacking malaria and one in every 3 people from dying for that same reason, thus avoiding more than one million deaths a year.

For the moment, as seen in the studies, it can be said that the effectiveness of the vaccine is maintained for at least 3 years, since the studies proposed for 5 years have not yet been completed. In case of need of reinsertion, with another dose of memory at 3 years of the first vaccination, the person would be immunized at another 3 years.
On the other hand, the vaccine obtained with clinical synthesis is a very low price. In this sense, one of the requirements that Professor Patarroyo proposed to make his vaccine available to the WHO was from there: That the total price of the 3 doses does not exceed 40 pesetas (2 pounds). In this way, Third World countries could use the vaccine. Other vaccines currently being investigated would be much more useful for people traveling to endemic areas such as passengers or tourists. In fact, the SPf66 vaccine has been created for the inhabitants of the Third World, who are precisely those who need it most.
Although all this opens the door to hope, we must not forget that research has not been able to resolve doubts about immunization.
- How long will vaccine protection last? Will we always have to go with a dose of memory?
- Is the new vaccine capable of providing immunity to very resistant slag of Plasmodium?
- Can there be a risk of disease destabilization and an increase in the mortality rate in areas and areas where malaria is in a stable phase after vaccination has begun?
At the moment it is very soon to answer all these questions. In any case, the work just published by the prestigious magazine “The Lancet” on this study values very positively the one carried out by the Colombian researcher Manuel Patarroyo. Geroa will say, repeating the words of Bitoriano Gandiaga to “see if he lives” or “if a smell of disappointment has come out through the window”.





