Possibility of EPO by encapsulated cells
Farmazian doktorea. Biofarmazia, Farmakozinetika eta Farmazia-teknologiako irakasle kolaboratzailea
Farmazia Fakultatea UPV-EHU, Vitoria-Gasteiz
Anemia is mainly due to the lack or total absence of a hormone called erythropoietin (EPO). This hormone promotes the creation of red blood cells. It secretes into kidneys, liver, uterus, muscle cells, endothelial cells and insulin-producing cells, depending on the degree of oxygen in the tissues. Precisely when the oxygen level of the tissues is reduced the regulatory factor HIF-1 flows, which considerably increases the production of EPO.
The secretion of EPO hormones in adequate amounts is critical. This is because if it is secreted less than it corresponds, the number of red blood cells in the blood is less than enough and, therefore, there is a risk that patients suffer a series of alterations such as fatigue, tissue hypoxia, less tolerance to sport and cardiac hypertrophy, increasing the size of the heart tissues.
The most effective option to treat all these alterations is to take EPO. Currently on the market is the medicine with human recombinant EPO. Patients with chronic anemia receive this hormone 2 or 3 times per week, approximately 1,000 to 9,000 units per week. On the other hand, a derivative of EPO has recently been published under the name of Darbepoetin: erythropietin. The main advantage of this new derivative is its duration. Its persistence in the organism is prolonged, so its frequency is much lower.
However, the importance and high interest of erythropoietin are not limited to anemia. Recent studies indicate that EPO may be effective in treating other chronic diseases such as thalassemia, arthritis, or myeloma. In addition, it may be useful in central nervous system disorders, such as Parkinson's or Alzheimer's disease, and in brain ischemia (when blood supply in the brain is interrupted) due to anti-inflammatory and neuroprotective properties.
Moreover, several studies have shown that the hormone EPO is able to modulate the intensity of the immune response and is also angiogenic, that is, promotes the formation of blood vessels.
Therefore, it is easy to understand the interest of many research groups and biotechnology companies to investigate the EPO molecule in depth and to develop new drugs with it. The aim of these efforts is to improve the properties of the hormone to effectively treat these diseases.
Muscle cells that secrete EPO
Given the properties and characteristics of the hormone EPO, it has begun to work on the development of a new strategy of administration of EPO in the laboratory of Pharmacy and Pharmaceutical Technology of Vitoria-Gasteiz. To meet this goal we have used microencapsulated cell technology. First, we have made muscle cells that secrete EPO, placed them in capsules of proper design and size, and finally donated the encapsulated cells to the animals.
The capsules protect muscle cells from the immune response. Keep in mind that these cells are foreign to the body and therefore destroy the immune systems. However, proper preparation of the capsules prevents the response of the immune system and, by protecting itself inside the capsules, the cells are able to flow the hormone for long periods of time. Consequently, they need to be “taken” less and treatment is more comfortable for the patient.
In addition, taking into account the angiogenic properties of the EPO and the ability to modulate the intensity of the immune response, tests were carried out using different forms of capsule administration. Although traditional intraperitoneal pathway was chosen, subcutaneous pathway was also tested with poor overall results in this technology. This last path is much more comfortable, so it would be of great interest – and a breakthrough for technology – if the capsules emitted by this route allowed to maintain in the long term the therapeutic effects of EPO.
Results and debate
The cells to be supplied were extracted from the mouse type C3H and later transformed into a laboratory for the secretion of EPO. These transformed cells were encapsulated and incorporated back into mice.
Ideally, more therapeutic products that flow cells, fewer cells and, ultimately, fewer capsules to incorporate animals for the same pharmacological effect. In order to achieve an ideal medium, a maximum of 300-400 cells were deposited in each capsule. Capsules of 480 micrometers were then prepared and EPO production of internal cells was measured.
To prevent any type of immune response in the animals after implantation, the capsules were carefully prepared. We made them with purified alginate, smooth surface and the same size. These characteristics are very important from the point of view of biocompatibility to avoid the response of the immune system.
We incorporate the capsules to two types of mouse, CH3 and Balb/c. As the cells came out of the first, they have the same genetic characteristics, but the second ones do not, so their probability of immune response is higher. Determining the dose to animals was a critical step. In fact, if the dose was excessive, there was a risk of toxic effects, including fatal effects of animals, while if it was too small there was a risk of not having therapeutic effects.
The first experiments were carried out in the C3H mouse, for which 20 mice were divided into 4 groups (one control group by way and another in which the capsules were collected by way). Animal hematocrit, a percentage of red blood cells in the blood, was measured for 100 days a week.
The results clearly show that animal hematocrit increased significantly in both groups, both in the collection of capsules intraperitoneal in mice and in the subcutaneous insertion of capsules. Hematocrit values were also higher than 80% at all times, so statistically significant compared to control groups.
However, to know the true value of microencapsulated cells it was necessary to provide and demonstrate their effectiveness to living beings that do not possess their genetic characteristics. To do this, we conducted the same previous experiment with 20 Balb/c mice and the results were statistically significant again in all cases: animal hematocrit remained above 75% during the 100 days of study.
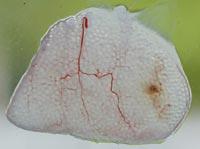
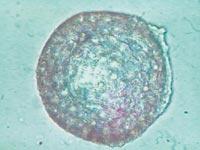
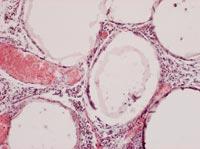
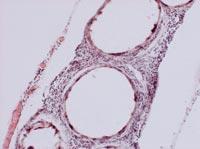
From all these data, we draw two main conclusions. On the one hand, the capsules were able to slow down or at least reduce the immune response of mice. On the other hand, the introduction of cells under the skin can be an opportunity to develop a chronic treatment with capsules.
To analyze the condition of the capsules, on 150 some animals of each group were killed and the capsules were recovered. The condition and structure of the capsules was different according to the route of administration of the cells. Thus, most of the capsules recovered intraperitoneally were isolated and few had superficial fibrosis caused by immune response. On the other hand, the capsules applied by the subcutaneous pathway constituted an added structure that had no indication of fibrosis by immune response. These structures were also full of capillaries, due to the ability of the hormone EPO to produce the growth of blood vessels.
In view of these initial positive results, we posed a new question: How long would cells deliver EPO with a single capsule administration and without using drugs that inhibit immune response? To answer this question we used the Balb/c mice again and the results were very significant. In fact, subcutaneous cells dried the EPO for 210 days. And that duration is the longest that has ever been achieved through that route. One of the explanations given by the hormone EPO itself to obtain positive results with different types of mouse and different ways of administration. In fact, the hormone EPO has the ability to promote blood vessels and plays various roles in the immune response.
In short, we can therefore affirm that encapsulation of cells that secrete this hormone is a suitable technological alternative for the chronic administration of EPO. Our next objectives will be to lengthen treatment and adjust the concentration of EPO and therefore hematocrit to physiological levels.
If you want to know moreOrive, G. and others 'Long-term expression of erythropoitein from myoblasts immobilized in biocompatible and neovascularized microcapsules' Molecular therapy Approved for publication Schneider, B.L. and other 'prevention of the initial host immuno-inflammatory response determinthe long-term survival of encapsulated myoblasts genetically engineered for erythropoietin delivery' Molecular therapy 7: 506-514. (2003). |