Decompression and overpressure accident

So far we have worked science through trials, that is, experimenting, but sometimes it can be very harmful that trials are conducted without knowing what science says. Therefore, today we bring to our section briefly the causes of the decompression and overpressure accident, so well known among divers.
Being at sea level, we endure an atmosphere of pressure. Moving 10 meters towards the bottom of the sea we will be under 2 atmospheres, 20 meters under 3 atmospheres, etc. Moreover, the concentration of soluble gases in a dissolution coincides with the increase in pressure. Thus, for example, in the case of 3 atmospheres, the concentration of gases that our body can accept will be approximately three times higher than on land, according to the Law of Boyle and Mariotte.
But where do the problems start?
When we finish the immersion and go up, the external pressure is decreasing. However, the air pressure we have inside our body does not decrease so quickly. Therefore, the air will try to remove it from the body by forming bubbles. If the bubbles form in the lungs, they can burst by overpressure. Bubbles can also form in the blood vessels, making blood circulation difficult and causing a decompression accident. To prevent this from happening, we need to replace our indoor air with a lower pressure air as we go up and give the body time for this process to take place in all the lungs and blood vessels.
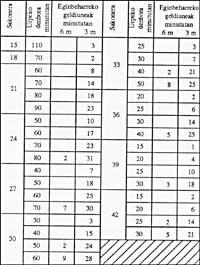
Attached is the following decompression chart that just in case can help.