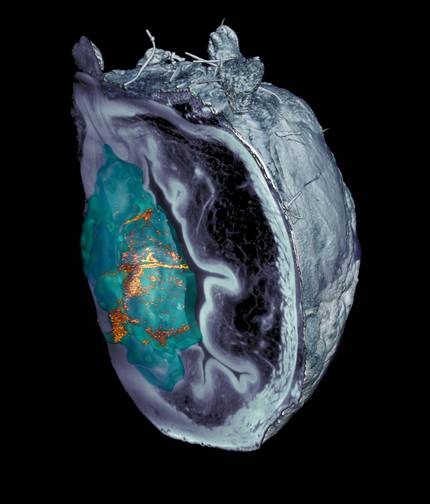
They've managed to reduce by 90 percent bladder tumors with nanorobots.
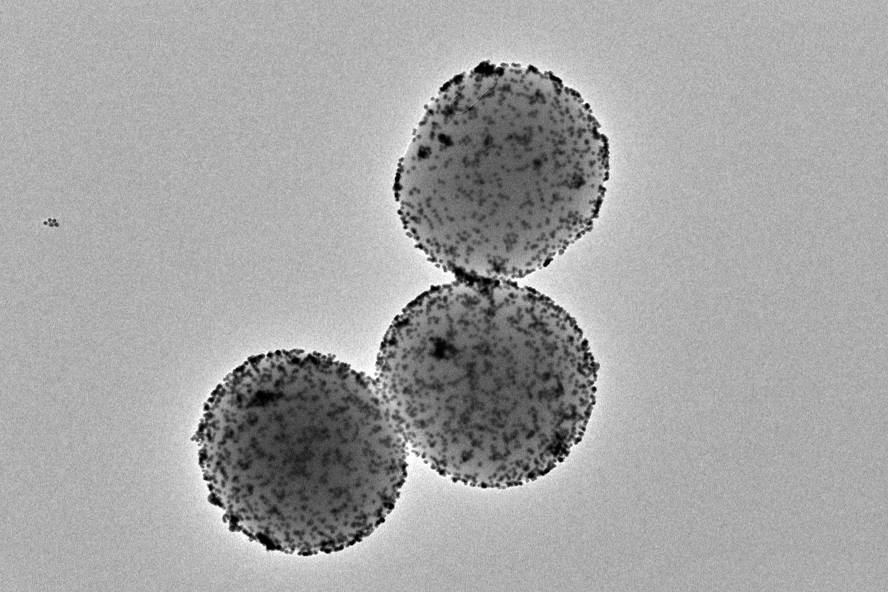
They've managed to reduce by 90 percent bladder tumors in mice, with a single dose of nanorrobots. These small nanomachines are propelled with urine water, they reach the tumor and are attacked with a superficial radioisotope. This work, led by the Catalan Institute of Bioengineering and CIC biomaGUNE, has been published in the journal Nature Nanotechnology.
Bladder cancer is one of the tumors with the highest incidence rate in the world and the fourth most frequent tumor in men. Although its mortality is not high, it is one of the most difficult to cure and almost half of the tumors reappear after five years. In current treatments, drugs are administered directly into the bladder, but despite good survival rates, the therapeutic efficacy is low.
Thus, the use of nanoparticles is a promising option since they are able to directly transmit the therapeutic agent to the tumor. Nanorobots stand out, nanoparticles capable of moving within the body.
The nanorobots used in this study have a porous silica sphere with several specific function components in surface. One of these is the urease enzyme, a protein that reacts with the urea present in the urine and gives the nanoparticle the ability to propel it. Another key component is radioactive iodine, a radioisotope frequently used for localized tumor treatment.
In the mouse study, the tumour volume decreased by 90% with a single dose. It is much more efficient considering that patients with these tumors often have to go to the hospital 6 to 14 times. The recurrence of these tumours after treatment is now being studied.
Even within the tumor
Previous studies have shown that the self-propulsion capacity of nanorobots can reach all bladder walls. This new work goes further by testing that nanoparticles move in the bladder and accumulate specifically in the tumor. In addition, they have seen that, in addition to reaching the tumor, they penetrate the inside, which increases the influence of the radiopharmaceutical.
The challenge was to clarify why nanorobots are able to penetrate the inside of the tumor. Nanorobots lack specific antibodies to recognize the tumor and usually the tumor tissue is more rigid than healthy tissue.
It has been observed that these nanorobots have the ability to decompose the extracellular matrix of the tumor, since they raise the pH locally by means of a self-propelled chemical reaction. So researchers have come to the conclusion that nanorobots collide with the urothelium like a wall is treated, but in the tumor, being more inflamed, they enter and accumulate in it.
In addition, localized administration of nanorobots carrying radioisotopes reduces the risk of adverse effects and the high accumulation in tumor tissue increases the therapeutic effect.
Researchers have explained that this work has opened the door to the use of other radioisotopes with greater capacity for therapeutic incidence.