Salsa of definition

Heavy means high density in its usual use. Metal means pure element or alloy of metal elements. The designation of heavy metal has been used for years, but raises doubts among experts. Is it necessary to define or classify metals? That is, do the elements that enter this sack have a certain collective character?
Noble gases, for example, are elements of group 18 of the periodic table. All of them are stable and chemically unreactive atoms. This gives them a certain collective character. The metals are elements capable of driving electricity and with metallic brightness. They can also form cations and contain basic oxides. All these characteristics are equal. However, perhaps this definition does not clarify much. In fact, according to this definition, most of the elements of the Periodic Table can be metals. Therefore, the metals are distributed in smaller groups, depending on their own characteristics and their use. Within this distinction are heavy metals, a name that has been used for years among chemicals, and which is still being used today, despite the uncertainties it poses. The International Union for Pure and Applied Chemistry (IUPAC) has not yet given its definition (it is a reference regulatory body in the field of Chemistry that establishes, among others, standards for the designation of chemical compounds). Moreover, he has published several reports on the debate on the definition of heavy metals.
No criterion without criterion
According to an IUPAC report, for years different criteria for the definition of heavy metals have been taken into account. Density, atomic mass, atomic number and toxicity have been used as criteria, among others. Thus, the bibliography contains several definitions. However, since the denomination has been used for years without any specific criteria, there are contradictions between definitions and others. The designation of heavy metals has also been used for semi-metals, such as arsenic in some cases. There is nothing clear.
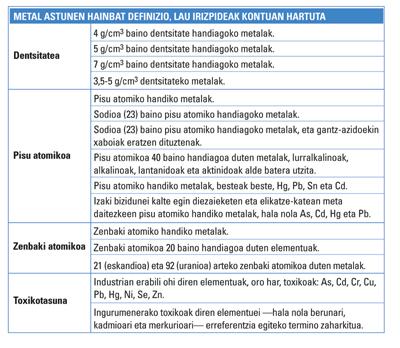
Curiously, the denomination of heavy metal was used until 1936 to designate large weapons or great talents or capacities. As for the chemical elements, one of the oldest references to the term appears in the third edition of the book Inorganic Chemistry, published that same year in London. However, this definition has not been used in subsequent publications. The definition given by Bjerrum took as criterion the density of the elements. In particular, heavy metal played density elements greater than 7 g/cm 3. Over the years this definition has been modified by modifying the density limit.
Although the classification by density was related to reactivity, some experts found that density was not of capital importance in the reactivity of metals. Therefore, the definitions have been adapted from the mass or atomic weight. This has somehow approximated the definition to the periodic table. That is, the best-known source of information on the classification of chemical elements. However, you do not believe that this criterion in function of mass is not clear either.
H. H. Bennet and R. J. According to Lewis editors, for example, heavy metals can be considered those elements that have an atomic mass greater than sodium (Na), that is, greater than 23, so those that are found from magnesium (Mg). G. G. M. M. For his part, Rand and his team affirm that heavy metals are elements with an atomic mass greater than 40, that is, from the scan (Sc).

Other definitions are based on the atomic number. Among them, at least a few experts or definitions agree: heavy metals are those with an atomic number greater than 20, that is, those ranging from sodium (Na). However, there is a small problem: in this group are included magnesium (Mg) and potassium (K), being the density of both very low. That is, it does not conform at all to the definition of density as a criterion. Not even from one of the lowest density limits.
Toxicity is another widely used criterion in the classification of heavy metals. Without another basis, due to its toxicity to the environment, some elements such as lead (Pb), cadmium (Cd) and mercury (Hg) are considered heavy metals, and their compounds.
However, the toxicity of heavy metals in general is not related to its intrinsic characteristics but to its concentration. What's more, often, the toxicity depends on the compound that forms it. For example, the characteristics of tin and tribulation oxide are very different. The toxicity of the first is very low, while the second is quite toxic. Something similar happens with chromium. Chromium is not toxic in stainless steel, while chromium ion relates it to lung cancer. This is, therefore, the reason why the concept of heavy metals is ruled out. In fact, it does not make much sense to introduce metals and their compounds in the same sac of toxicity.
Obsolete term

Eleven definitions, many doubts and nothing clear. That is, without a clear collective character for heavy metals. In fact, despite the common characteristics of metals, each one has its physical-chemical characteristics. Not only that, each of them can form several compounds, each of which has its own characteristics. Therefore, it makes little sense to include metals in the same group and their compounds.
Therefore, according to the IUPAC Expert Committee, it would be convenient to do without the concept of heavy metals, since it has no scientific basis. In addition, at present, as mentioned above, the concept of heavy metals is often associated with a group of elements or compounds that may be related to pollution and toxicity. However, this group of experts makes no direct relation between density and the rest of chemical physical characteristics (atomic mass, atomic number, etc.) and toxicity.

Therefore, from an academic point of view, it can be clearly said that the concept of heavy metals is obsolete and should be discarded. However, it will be difficult to extend this view of the academy to society, and even achieving it, there would still be a problem with the denomination of metal. In fact, the term metal is currently used to refer to both metals and their compounds. This means that metals and their compounds are treated as being of the same physico-chemical, biological and toxicological characteristics, and this is not so.
The opposite occurs in the case of carbon. That is, the designation of carbon is not used to represent all the compounds of carbon. In fact, if this were so, carbon would be considered a human being carcinogenic, since some of its compounds are carcinogenic. Therefore, metals, like carbonic compounds, should be analyzed separately.