Debt with relativity
Although the speed of light is unsurpassed, the relativistic effects associated with its proximity have a direct impact on everyday life. These effects are, for example, the main ones responsible for the color of gold, so appreciated in the world of jewelry, and the liquid character of mercury used in thermometers. What are those relativistic effects and what relationship are there between those effects and those distinctive features of the aforementioned metals?
300.000 km/s, is an immense speed. To put some examples, at that speed, in just a second we gave the world seven laps, or the distance from the Moon to the Earth would be formed in almost a second. Undoubtedly, these speeds are not in our hands. For example, Curiosity parking recently reached Mars, at an average speed of 12,000 km/h, which means 90,000 times the speed of light.
However, in the atomic world there are particles that approach this speed up to the consequences that it entails. Thus, in the case of heavy atoms such as platinum, gold, mercury or uranium, the high positive charge that accumulates in the nucleus makes electrons closer to the nucleus move very quickly, near the speed of light.
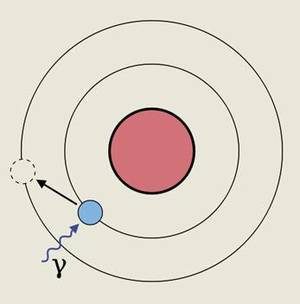
As can be deduced from Einstein's theory of relativity, in these cases the mass of the electron increases, being this the main source of the relativistic effects that are seen in the case of heavy atoms. This is because by increasing the mass of the electrons that are close to the nucleus, their orbits decrease. This, in turn, causes the contraction and expansion of some of the superficial orbals involved in the chemistry of an atom and the modification of the energy level of the electrons contained in them. This allows to increase or decrease the energy level that electrons must absorb to move from one energy level to another.
The contraction and expansion of surface orbitals of heavy atoms, as well as the consequent change in the energy level, result in properties of heavy elements that would otherwise not appear.
The color of gold
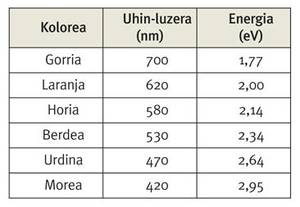
One of these features is the natural color of gold, so valuable in jewelry. This is because, in this case, these expansions and contractions of surface orbals make an electron of the surface pass from one orbital or energy level to another. In fact, the energy that must absorb this electron to make the above-mentioned leap is from the order of 2.7 eV, a value that is found between the energies of purple light and blue.
To understand the yellowish of gold, it must be taken into account that this color is complementary to violet, that is, if we remove the purple component of white light, we see yellow and vice versa.
Therefore, when we radiate gold with white light, this absorbs the photons between blue and violet, and we see the rest of the light reflected on that golden surface.
But what is the relationship between all this and the relativistic effects? Let us analyze, therefore, the energy interval corresponding to this jump from one orbital to another in lighter elements such as gold, that is, in the case of copper and silver placed on the gold in the periodic table. Thus, in the case of copper, this energy range is around 1.4 eV, while in the case of silver it is around 3.7 eV. Note that both values are outside the energy range of visible light. In the case of copper and silver, the relativistic effects are not so important, but if there are no, as we go down in the periodic table, we would have to increase the energy range corresponding to that jump. Thus, if these relativistic effects did not exist, the electron mentioned in the case of gold should absorb more energy than in the other two cases to make that leap. In reality, however, the relativistic effects make the energy or orbital levels that come into play in this leap are closer to what they should be, placing their separation between copper and silver. Coincidentally, for the enjoyment of our eyes, in the case of gold, that energy jump is close to the energy level of the purple light, and we perceive the yellow color.
Liquid mercury liquid mercury
Another significant example is mercury. This metal, used in measuring devices of temperature and pressure, is strange, unlike other metals, since it is liquid at room temperature. And this nuance is also a direct consequence of relativistic effects.
In fact, the contraction of the last orbital surface of the mercury occupied by two electrons makes the electrons contained there strongly bound to the nucleus more strongly than those which could be found in the absence of relativistic effects. The effect of contraction of this orbital on this characteristic will be more clearly seen if we compare the ionization energy of mercury, that is, the energy necessary to remove an electron, with that of cadmium and that of zinc, which are lighter than their pairs. Thus, taking into account that the ionization energy of zinc, located two lines above, is 9.42 eV and that of cadmium, just above the mercury, is 9.03 eV, it should be expected that the mercury energy is inferior to these two values. However, the contraction of the aforementioned orbital causes the ionization energy of mercury to be of the order of 10,51 eV, much higher than that of the other two of its group.
To understand the relationship with all this, the fact that mercury is liquid at room temperature, it is worth remembering the structure of solid metals. Thus, when in solid state, the metals release the remaining electrons and create the bands of electrons corresponding to all atoms. Therefore, in a metal the nucleus and the inner electrons are located at a given point and the liberating electrons have the freedom to move through all the metal around those specific points. This structure is the main responsible for the high electrical and thermal conductivity of metals.
In the case of mercury, on the contrary, the contraction of the last occupied orbital prevents the existence of this structure, since the two local electrons cannot remain free, as would happen if this relativistic effect did not exist. Consequently, mercury is in liquid state at room temperature.

...and much more
However, the yellowing of gold and the liquid character of mercury are just two examples of the consequences of relativistic effects. Beyond these two cases, these effects greatly condition the chemistry of heavy elements. Thus, the relativistic effects make it possible for these heavy elements to react differently. Likewise, the different chemical behavior of the lighter heavy and assimilable elements (platinum/palladium, gold/silver, mercury/cadmium...) is partly due to these effects.
Therefore, although the speed of light seems a matter of fiction, the effects related to it are very present in our daily life. Therefore, we might say, to some extent, that human beings are in debt with these relativistic effects.