Thalidomide, 50 years later
Interest in a banned drug is increasing in recent years. In fact, researchers are showing that this drug, thalidomide, is effective in treating complex diseases. Thus, the FDA, the U.S. Food and Drug Administration, authorized in 1998 the marketing of thalidomide for the therapy of nodosum erythema associated with leprosy. Subsequently, it has been authorized for the treatment of multiple myeloma and has been done by the European Medicines Agency, EMEA, in 2008. However, permits have been granted with strict rules of use.
In addition to treating leprosy and myeloma, researchers believe thalidomide may be useful in other diseases, so tests are being done to assess its effectiveness. Among others, they have obtained good results in systemic and discoid lupus erythematosus, as well as in dermatological-rheumatological diseases such as foot and mouth disease and Behcet disease.
It is also being investigated if it can be effective in treating more known and widespread diseases: Chron's disease, rheumatoid arthritis, ulcerative colitis, some cancers such as prostate and kidney cancer, some aids-related alterations such as diarrheas and aftose ulcers...
However, both in therapy and in clinical trials, doctors and researchers should take into account the side effects of thalidomide, as they are many and varied: drowsiness and fatigue, decreased white blood cells, anemia, tingling and excretion of arms and legs, skin rashes, dizziness... And in no case can they forget their influence on fetuses. In fact, thalidomide causes malformations and high-dose abortion.
Dark past
Precisely because of its effects on fetuses, thalidomide has been marginalized for many years. Thalidomide, born on Christmas Day 1956, was the first child with malformations. The thalidomide, produced by the German company Chemie Grünenthal, was the daughter of a company worker, who distributed the pills free of charge to his employees, who were removed by his wife, pregnant and were suitable to relieve insomnia, anxiety and nausea during pregnancy.

However, no one thought then that the thalidomide was responsible for the malformations. Chemie Grünental tested the drug on animals for two years -- apes, rats, rabbits and dogs -- and, according to the report that shows the results, no negative consequences were detected for pregnant females or newborns. Thus, the German government had no trouble granting the marketing authorization of thalidomide, which was put on sale in Germany in 1957 with the trade name Contergan.
In the United States, thalidomide did not obtain marketing authorization, considering that evidence to ensure safety had been underperformed. In many other places it did not suffer discomfort; with the help of advertising it was divided into 50 countries with 80 trade names, being in a short time the third best-selling medicine in the world.
At the same time, children with severe malformations were born in the countries where thalidomide was sold. Some had totally distorted the muscles of the face; others, arms or short legs; or they had no ear; or they were blind; others with alterations of the heart, of the digestive system or of the kidneys... In total, it is estimated that in the 7 years in which the drug was available to pregnant women 20,000 children were produced with these consequences. Of these, 40% died before the first year.
Doctor Widukind Lenz was one of the first to discover the direct relationship between thalidomide and malformations. In 1961 he published an article in the medical journal The Lancet, entitled "Thalidomide and congenital abnormalities". He was subsequently removed from the thalidomide market. In some countries it was immediately removed, for example in Germany and Britain. In other cases, however, measures were taken later, including in Spain: It remained on the market until 1963.
Illuminating the mechanism

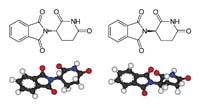
The researchers relatively quickly discovered that one of the two talidomized isomers was harmful and the other beneficial. In fact, the molecule adopts two equal forms, but one as a mirror of the other: R and S. R-thalidomide is good and S-thalidomide has harmful effects. Synthesizing and taking only the R isomer does not solve the problem: inside the body, one becomes the other.
However, other aspects of thalidomide remain unknown. Researchers know that the mechanism of action of thalidomide is complex and multifactorial, since with a single mechanism they cannot explain its anti-inflammatory effects, regulators of the immune system, etc., but for many of them they do not have a precise explanation. And they are not yet clear about the mechanism by which it causes malformations in the fetuses.
But they are working. Recently researchers from the Tokyo Institute of Technology have taken a step on this path. Takumi Ito and his team have published in the scientific journal Science that thalidomide is associated with a protein called cerebon, which causes malformations in zebrafish limbs and chickens. According to the article itself, the research "will serve to make a non-toxic version of thalidomide."
Experts, attention
The doctor of the Hospital San Agustín de Santiago Pintado Avilés closely follows the investigations on thalidomide. In fact, last year he published an article about the disaster that this drug caused 50 years ago in the medical journal Jano ("The catastrophe of thalidomide in the fiftieth anniversary of its marketing"). Of course, the Takumi Ito team has received with great interest the news of the research carried out.

According to Pintador, the final objective of the researchers would be "a great step forward", to develop a non-toxic thalidomide. In his opinion, the new applications of thalidomide are "hopeful"; besides fetal malformations, their side effects are reduced compared to chemotherapy. Therefore, he advocates the use of thalidomide in therapy, "always with the guarantee that the necessary measures have been taken to avoid all risks in patients in reproductive period". However, not enough progress has yet been made: "We have to do more research, we have to study more cases and let the time pass."
UPV Organic Chemistry researchers Claudio Palomo and Antonia Miel have also learned about the work published in Science. In his opinion, although this research may help, "it must be noted that many other proteins also interact with thalidomide." In addition, they warn that the effects of thalidomide vary from species to species: "for example, it generates malformations in people, chickens and fish, but not in the mouse." So match the last words of Pintador: "We must continue to investigate."






