Smoga: smoke cloud
Between 5 and 10 December 1952 a special climate situation occurred in London. During those days the air layer over London barely moved and remained in the same place. The cause was the seasonality of the cold air layer formed near the ground, the temperature inversion. Normally, the air near the ground is warmer than the surface, lighter and ascends by convection. Colder than the above makes it not rise and if there is no wind it remains.
In Amartea, when it was cold, the heating systems of London households were working on fire and emitting thousands of tons of smoke and pollutants into the atmosphere. Fumes and pollutants had no air dilution device and concentrated on this layer of cold air in Upper London. Consequently, the most intense smog ever known was formed. In broad daylight only 1-5 meters could be seen. In those five days of December he killed more than 4,000 people, mainly elderly people and children.
New event?
The word smog is a new word formed by contractions of the English words smoke and fog, which perfectly explains its meaning. In fact, smoga is a cloud formed by smoke.

In London it is not a new event and it is already the XVII. In the chronicles of the twentieth century they complained of the "stain" that covered the city. However, as a result of the industrial revolution (in short, the steam engine), the frequency and consistency of smog increased in the 19th century. throughout the 20th century. Moving away from the industrial center and expanding the city mitigated the problem of smog. However, after World War II, the weak economic situation resulted in the use of low-quality coal (high sulfur content) for heating. In parallel, smoga was compacted and proliferated.
How is smoga?
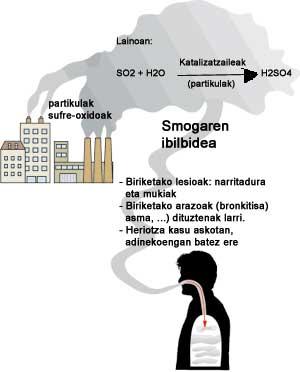
By burning coal or any fossil fuel we would theoretically obtain carbon oxide and water vapor. However, coal has dirt (such as sulfur or parts that are not burned) or does not burn well (due to oxygen shortage, for example) and other products are formed in rivers. Impurities will form sulfur oxides, mainly sulfur dioxide, ash and carbon dioxide oxygen deficiency.
Sulfur oxide (IV) reacts with atmospheric vapors and drops of water, partially catalyzed by ash particles, condensing in the form of sulfuric acid into ash particles. Consequently, we have the drops of diluted sulfuric acid, a kind of acid rain.
Conclusions
Sulfuric acid is very corrosive, as anyone who has studied chemistry at school knows, and that is why it is also harmful to things and people. It attacks stones, metals and fabrics, shortening the life of buildings and other infrastructures. It also affects man, especially the respiratory system.
When breathing smoga, the bronchi become irritated. A lot of mucus occurs and the bronchi become inflamed. People with bronchitis, asthma, or other lung diseases aggravate and aggravate the problem. Mucus and inflammations prevent breathing. Sometimes the airways are blocked by the mucus, which causes the death of the patient, both drowned and cardiac. This happened in London in 1952 with some 4,000 people. Most were between 45 and 64 years old.
The death chain lit the alarm light of health officials. A comprehensive study was conducted and the conclusion was clear: if it was intended to be avoided, it was necessary to greatly improve the quality of urban air. Strict anti-pollution measures were taken (the Clear Air Act passed in 1956) and Londoners have forgotten the old dense and suffocating mists. The switch to gas and electric power for heating and the renovation of the old facilities were the most effective measures.
Photochemical smog The Clean Air Act eliminated the risk of smog from London's atmosphere. However, the air in London and other cities (Mexico City, Los Angeles, Santiago de Chile...) is not as clean or innocuous as expected. Smoga has not disappeared, although it has not been a striking aspect of yesteryear. It has been transformed and its origin is another. However, it can be as lethal as before. Photochemical smog, the main pollutant in our cities, originates from heating systems and vehicles. The source is cars, trucks and buses. In internal combustion engines, fuel combustion (gasoline, diesel...) should only theoretically form carbon dioxide and water vapor. However, due to combustion conditions nitrogen oxides are also generated (considering that the air entering the cylinder has 70% nitrogen), along with poorly burned organic products. One of the nitrogen oxides, nitrogen oxide, is a powerful lung irritant that has replaced sulfur dioxide as the main pollutant in our cities. In addition, nitrogen oxide (IV) also reacts with water forming nitric acid. But nitric acid is not the greatest risk of this smog. The chemical sauce that emerges from the exhaust pipes of the vehicles is not maintained. Solar ultraviolet radiation produces chemical reactions inside this sauce, resulting in other chemical compounds. That's why it's called photochemical smog. The really dangerous contaminants are the secondary ones, mainly volatile organic compounds and ozone. Many of the volatile organic compounds, such as benzene, are violent carcinogens. On the other hand, although ozone protects us from the ultraviolet rays of the sun in the stratosphere, on the earth's surface it is very harmful: it prevents breathing, damages trees and crops and runs many materials. The Parthenon of Athens is suffering terrible damage from the photochemical smog of the city, which has not occurred for 2,000 years, and which can be carried out in the short term. In some places the problem is very serious. For example, in 1990 in Mexico City, the ozone concentration exceeded in 310 days the maximum value recommended by the World Health Organization. The problem has only one solution: to limit traffic. Look! |





