Mössbauer spectroscopy
Rudolf Ludwig Mössbauer (see figure 1) was born in München on 31 January 1929. Therefore, this year marks the 60th anniversary of his birth. The story of the effect that bears his name is very interesting. Free announcement While young Mössbauer was conducting experiments for his doctoral thesis, he discovered a strange phenomenon that he did not expect. In these experiments he used the isotope 191Os as a source. This isotope, due to the beta disintegration, passes to the 129 keV state of the isotope 191Ir, moving from this situation to the fundamental situation, that is, when moving to the state of minimum energy emits a gamma ray of 129 keV, with a line width of 5x10-6 eV.
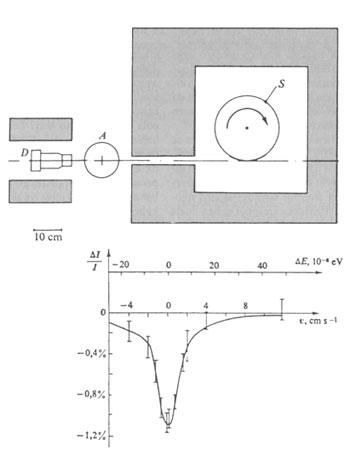
Mössbauer measured the transmission of these gamma rays through the natural iridium crystal (38.5% 191Ir) and built the device indicated in Figure 2. This experiment is called resonant fluorescence or absorption. The gamma rays emitted by the nuclei of the source when passed to fundamental state in this type of experiments are absorbed by cores of targets of the same type, rising to the same initial situation as the emitting nuclei of the source without the direct transmission of that gamma ray. Fluorescence was well known in atomic spectroscopy. If the radiation is of nuclear origin (because the energy width of the radiation is very small), the delay energy that supports the nucleus by emitting gamma ray is greater than the natural width of the line, so no resonant absorptions (or fluorescence) occur. If the Doppler effect brings energy to the gamma ray, resonant absorption will occur.
This can be done mechanically, giving speed to the source (see figure 2) and thermally, increasing the temperature. Therefore, at high temperatures, the Doppler effect of thermal origin generates a sufficient width in the emission and absorption lines to overlap each other, so that resonant absorption occurs. That was what the young Mössbauer expected, that by increasing the temperature would increase the resonance, as was seen when using steam instead of glass. Mössbauer, for his part, found that by lowering the temperature the absorption increased.
After his observation, he began his work, both experimental and theoretically, to clarify this unprecedented effect. When the core is attached to a crystal, its movement can be compared to the movement of a group of oscillators, which is what Mössbauer did. In this case, if the delay energy is insufficient to move from the fundamental situation to the first (creation of phonons) (conservation of the linear moment), the entire crystal (and not only the emitting core) will be delayed. Therefore, the delay energy will decrease according to the number of cores of the network (order 1010 in the network of a cubic micron) and being this value much smaller than the width of the energy line of the gamma ray, will be negligible. In these cases we speak of emission without delay.

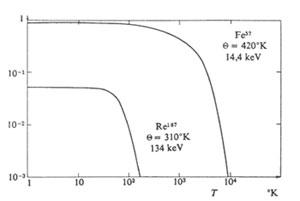
Mössbauer estimated that, by replacing the crystalline grid with frequency oscillators\ E (following Einstein's model), the lag-free emission will only occur if h\ E=k\E>>E>2/mc2 (E ray energy{= Einstein temperature). However, being the real spectrum of vibrations more complex than the one exposed in Einstein's model, he later used Deby's approach. This model found that by raising the temperature the probability of emission without delay decreases and that if the temperature is high with respect to the temperature of Deby the probability is negligible (see figure 3). In the same figure, if the temperature of Deby is increasing, that is, if the binding forces of the crystal are increasing, it is also indicated that the probability of emission without delay is destroyed at higher temperatures. Mössbauer exposed the characteristics of the effect in 1958, but spent more than a year until its approval by the scientific community. For his work he won the Nobel Prize in 1961.
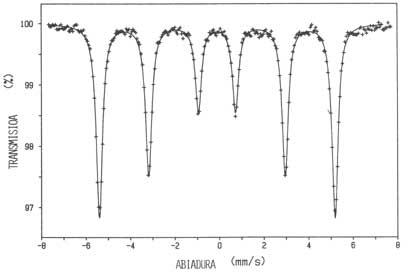
The nuclei of Mössbauer, that is, the nuclei that emit gamma rays without delay, are many, being the best known the 57Fe, since the probability of events without delays at room temperature is relatively high and iron is part of many substances of interest. The incomparable precision of the Mössbauer spectroscopy is that the natural width of the line is 1013 times less than the transition energy, that is, compared to the aforementioned precision, we can measure the changes in the order of a micron at a distance from Earth to the Moon.
The energy variation corresponding to the Mössbauer spectrometers is made by the relative movement between the sources and the absorbers, that is, by the Doppler effect. Speeds are usually small, several millimeters seconds.
The electrical and magnetic interactions in which atomic nuclei intervene are called hyperfine interactions. These interactions are easily observed in the Mössbauer spectroscopy, since, as mentioned above, the Mössbauer technique is high-resolution. The study of the hyperfine interactions indicated below allows to know the state of the absorbent core and its immediate environment.
a) Isomic variation or interaction between the non-punctual distribution of nuclear charge and electrons likely to be in the nucleus. This interaction generates a variation of energy levels. Since this change is not the same in the fundamental situation and in the exciting situation, that is, in the one with the greatest energy, the absorption line slides. The intensity of the slide gives us the idea of the density of electrons likely to be in the nucleus or the shielding in them existing, that is, of the chemical medium of the nucleus of Mössbauer.
b) Quadrupolar multiplication or interaction of the electric quadrupolar moment of the nucleus with the gradient of the electric field in which the nucleus is located. This interaction generates a multiplication proportional to the crystalline field. It therefore describes the symmetry in the Mössbauer core environment.
c) Magnetic multiple or interaction of the magnetic dipolar moment of the nucleus with the magnetic field of the nucleus position. This multiplication gives us the measurement of the local field of magnetically arranged media (see figure 4).
Apps
In the case of chemical research the most important Mössbauer parameters are given in the isomic and multiple quadrupolar variation. These parameters carry out studies of spin and oxidation states, molecular symmetry, analysis and separation of chemical compounds, corrosion, catalysis, etc.
Research into the magnetic properties of materials is the most common application of Mössbauer spectroscopy. The most commonly used Mössbauer isotope is 57Fe, as iron is one of the most common components of magnetic materials. Much of the information on magnetic compounds can be obtained from hyperfine parameters and some of the investigations that can be carried out are: Temperature and type of magnetic order (ferromagnetic, antifromagnetic, etc. ), distribution of atoms between magnetic or crystallographic positions not equivalent, study of the magnetic structure, etc.
Although Mössbauer spectroscopy is a very novel use of metallurgy, some of the essential features have already been limited and very special experiments have been conducted. Some of the research that can be carried out through Mössbauer spectroscopy are: quantitative analysis of solid solutions, martensitic changes, precipitation, amorphous alloys, defects, etc.
Several biological molecules carry iron and other metals and the Mössbauer effect is very useful for studying some enzymes and proteins, one of the most remarkable cases being hemoglobin. In biological compounds, sample preparation is special, must be in aqueous dissolution and measured when frozen. The most studied molecules are hemoproteins, proteins that contain iron and sulfur, and those that accumulate and transport iron.
Mössbauer spectroscopy has also been used in other areas, some of them Mineralogy, Geology (terrestrial and lunar), Archaeology, non-crystalline matter, etc. being.