Aluminium around us
From the design point of view, the choice of material for the realization of a part or component depends on different variables. To facilitate the analysis of these variables will be considered divided into two groups. The first group takes into account a series of conditions related to the characteristics that the part should have (mechanical, electrical, corrosion behavior, etc. ). In the second, those related to the price of the material will be considered. Taking these two groups into account, the most suitable material is chosen for each case.

Compared to what happens daily, perhaps what was said before is too simple. In many cases the processes of obtaining the material, its characteristics and price are related. Some of these examples are found in different types of composites. Because the procurement processes are still very special, there are composites that are too expensive, so their use is quite limited. On the contrary, if these products are increasingly used, the price of the material decreases by increasing production. In addition, although the technological characteristics of the same are maintained without improving, with the decrease in price competition against other materials will be increased. As you can see, the process is cyclical.
What is happening right now with compotes is not new. The same happened with other materials and the case of aluminum can be considered as an example of this. This article will take into account the evolution of aluminum. This example will be of great help in understanding the imbalances between the hope placed in new composites and materials and their still scarce use.
From kitchen utensils, windows, parts and components of the car and other transport systems to drinking cans, it is virtually impossible not to use aluminum parts throughout the day. However, aluminum is not a long used material, as its history began in the middle of the last century.
Aluminum does not remain free in nature and was not known until the beginning of the last century. In the 1920s the German researcher Friedrich Wöhler obtained the first aluminium blood cells. However, it had not yet broken the path technologically and took 30 years to market it. In 1854, the French chemist Sainte-Claire Deville began to commercially obtain aluminum bars by treating aluminum chloride with potassium (which was replaced by cheaper sodium) and the following year was presented as a new product at the Paris Exhibition.
Due to the procedure used by Deville aluminum was very expensive and at that time that new material was considered silver. This caused that in the next 30 years the annual world production did not reach three tons. At that time the problems of producing more quantity were not technological but economic. The price of aluminum was so high that its use was very limited. However, at that time the researchers considered it to be a material of great future (this story is being repeated with new composites and alloys).

To lower the price of aluminum we had to invent another path of achievement, which was not done until 1886. That year and independently, the American Charles Martin Hall and the French Paul Louis Toussaint Héroult simultaneously invented an electrolytic procedure to obtain aluminum. Aluminum oxide (alumina) dissolved in the mineral called crriolite, is decomposed by the application of aluminum and oxygen at high temperatures by an electric current. The Hall/Héroult procedure has been used to date and, as is evident, the importance of electricity is very high. Therefore, the development of aluminum depended on the evolution of another type of technology. It was not possible to consider the electrolytic procedure until electricity could be used cheaply and abundantly. Currently, to obtain a ton of aluminum it takes between 15 and 18 MWh.
This new procedure allowed a significant reduction in the price of aluminum (see figure), although the sale was very limited. Hall set up a factory in the city of Pittsburgh to produce aluminum, but soon realized that it was building up in the factory without selling the metal. At that time the best customer was the Pittsburgh steel company. The technicians of this industry realized that the characteristics of a liquid steel improved with the addition of aluminum. However, as a ton of steel requires very little amount of aluminum, it is evident that with this use the aluminum market was quite small (it seems surprising that steel was one of the first in aluminum; now in many respects there is a strong competition between steel and aluminum (of course, aluminum alloys).
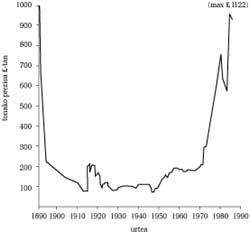
Consequently, the cheapening of the metal by modifying the obtaining procedure was an indispensable condition, but nevertheless it had not been confirmed that this would expand its use. To do this, we had to look for new markets and respond to the new technological problems that arose in each case.
To create new markets, the characteristics of aluminium had to be taken into account. The most important characteristics are: low density (it is considered a light metal), good corrosion behavior, good electric conductor, suitable for forming (that is, to give special shapes) and good mechanical resistance alloy with other metals (in most applications aluminum is alloyed with other elements).
Taking these characteristics into account, it is easy to understand the diffusion of aluminium XX. It is related to the new technologies of the twentieth century. Its density is low, so from the first moment of the aircraft fuselage appeared as suitable material. Thanks to its electrical characteristics and density, the high-voltage air cables used to transport electricity are made of aluminium. Therefore, the diffusion of the use of aluminium cannot be understood without the development of other technologies or advances.
Aluminum development technology (new alloys, heat treatments, bonding procedures, etc.) has been largely related to aeronautics. In this field this material has been dominated until recently (looking ahead there are increasingly available composites). However, the diffusion of aluminum in other transport systems has not been so wide. An example of these is that of cars.

As mentioned above, the emergence of a new application often generates new technological problems. This happens when it comes to making in aluminum most of the parts and components possible to reduce the weight of the car. That is, to reduce weight you can not directly make the piece that until now was made of aluminum steel, although the right aluminum alloy has been chosen to maintain the resistance. Outstanding issues include sheet metal forming procedures and tools, final surface quality, welding between different materials and corrosion. Considering all this, Honda recently released a special version of the NSX. In the traditional version the percentage of aluminum did not exceed 7%, but with the aim of reducing weight, with the use of aluminum in the body the percentage of this material rose to 31%, thus decreasing the weight of the body in 140 kg.
For the release of this version a new body design was developed, taking into account that the mechanical characteristics of aluminum (i.e., aluminum alloys) are very different from those of steel (strength, rigidity, difficulty). In addition, the forming of steel plates is very worked. On the other hand, the formations of the aluminium plates are relatively new (the extrusion of aluminium is well mastered, but the same does not happen with other obtaining processes), so the forging of aluminium before building the car was studied in depth by Honda. The same can be said for other problems (final sheet quality, dimensional accuracy, welding, etc. ).

After the launch of this new aluminum car, Hondako technicians assure that there are still many problems to solve. The forming facilities of the steel plates cannot be compared to the characteristics of aluminium and in order to compete in the field of production it is necessary to adapt the forging equipment (machines, dies, lubricants, etc. ).
However, the biggest problem of aluminum for use in car bodies is not technological but economic. The price of steel plates compared to aluminum is much cheaper. Therefore, in this case it is also as with other previous applications. Consequently, in the coming years there does not seem to be a significant change in this area.
Finally, we must take into account an important feature that has not been highlighted until now: aluminum is a recyclable material. As a consequence of the mandates of the European Union, in some cases the selection of materials is made taking into account its recycling capacity. From this point of view, aluminum is a very appropriate material. After collection in landfills, due to its low melting temperature (which does not reach 700ºC), the use of aluminum scrap is relatively cheap. On the contrary, compared to steel, magnetic metal is no more difficult to collect in landfills.

In short, the evolution of aluminium used most among metals after steel has been taken into account throughout the article. Although in daily practice many parts are aluminum (or even in some components), their development depends on other technologies and factors. Among other things, we must mention the variations in the price of energy (due to the large amount of energy needed to obtain aluminum, the increase in the price of it makes the price of aluminum considerably increase).
In other cases, the use of aluminum implies changes and rethinking from the point of view of the design of the part and the procedures of elaboration of the material. As seen in the car example, a lot of work is needed. To a large extent, the same is happening in recent years with new materials called composites. With composites, above all, but the use of ceramic materials requires in many cases the adaptation of equipment and designs in the field of production, which necessarily requires a time.
Aluminium applications Electrical applications: its electrical conductivity is 65% compared to copper, but its lower density and price makes it the most suitable material to form long distance electrical networks. Currently, half of the electricity is transported through aluminum. Chemical industries: their corrosion behavior is very good, so it is a food processing and packaging material, cheaper than stainless steel (in many cases it is considered that the most important use of aluminum is aeronautical and so it is from the point of view of the characteristics of the material. However, half of the production of Alcola, the world's largest producer of aluminum, is intended for the manufacture of drinking cans. Structural applications: the strength/weight/ratio of some aluminum alloys is very good for replacing other types of materials in structures. In addition, its corrosion behavior makes maintenance costs very low. Alloys Al/Mg/Si and Al/Mg/Si/Mn are used for these applications. On the other hand, special geometries can be obtained through extrusion, such as special sections used in aluminium windows. Inside this group can be considered windows, balcony railings, screens, etc. Transportation: in the construction of trains, both in structures and in different internal components (doors, storage bars,...), aluminum has long been used due to its low density. In cars aluminum is also used to reduce weight on certain components (pistons, carters, etc.) and in other applications because its thermal conductivity is good (blocks, cylinder heads). However, although the value of its density is very interesting, replacing steel in the bodies to be the main material, it is still not considered a real alternative. Aeronautics: the development of aluminium is largely due to the aviation industry. Copper alloys, whose mechanical strength is high thanks to thermal treatments, have been used very frequently in aircraft. However, in the coming years some composites can replace a large number of aluminum aircraft. However, in the last decade new Al/Li alloys are being developed, which allows aluminum to maintain its importance in this field (the European Airbus 320 aircraft, in addition to conventional high-strength aluminum alloys, is equipped with new Al/Li alloys). |





