CIC bioGUNE deepens the basis of neurodegenerative diseases
A new research by CIC bioGUNE opens a way to broaden knowledge about amyotrophic lateral sclerosis (ALS) and similar neurodegenerative diseases.
For the first time, the structure of the protein Vps54, a protein of four groups of proteins called GARP complex, has been resolved. In mouse models, specifically in the mouse called wobbler, this protein appears mutated, which causes neurons to gradually degenerate.
"Probably many of the patients affected by a motoneurodegenerative disease will not have the same mutation as wobbler mice, but it is possible that, on the other hand, some patients have a lower level of complex GARP that can cause the disease," said research chief Aitor Hierro.
The study carried out has allowed, for the first time, to solve the structure of this protein in the region where the mutation occurs. In the experiments it is confirmed that the mutation destabilizes the protein Vps54 and thus the complete GARP complex.
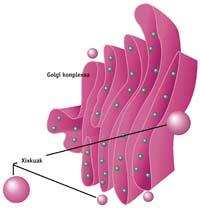
The GARP complex participates in the transport of components between cell organelles, specifically in the recycling process of acid hydrolase receptors. Acid hydrolase are enzymes that digest proteins in lysosomes (cellular stomach). The receptors of acid hydrolase are collected in the vesicles and are taken to the Golgi complex to recycle. The complex GARP physically joins the Golgeo.
According to this study, the levels of the GARP complex in wobbler mice would decrease considerably, not working properly the conduction of the acid hydrolase receptors to the recycling of Golgea.





