A fifty carbon cage
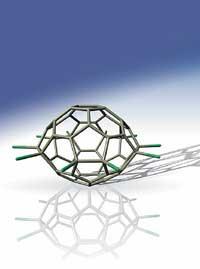
These spherical caged molecules do not have a certain number of carbon atoms, but the most stable are sixty-seventy atoms. Even greater than these have often been synthesized, but never fifty.
The difficulty lies in stability. Fulerenes look like a football, that is, in these molecules carbon atoms are interconnected forming hexagons and pentagons. In the most stable “balls”, for example in a sixty carbon structure, each pentagon is surrounded by five hexagons, but in those of fifty carbons this rule is broken and therefore very unstable.
Therefore, the molecule obtained by Chinese chemists is very unstable. And although this feature poses problems, it can also have very interesting applications such as nanotechnology.





