Design of metal-free organic batteries
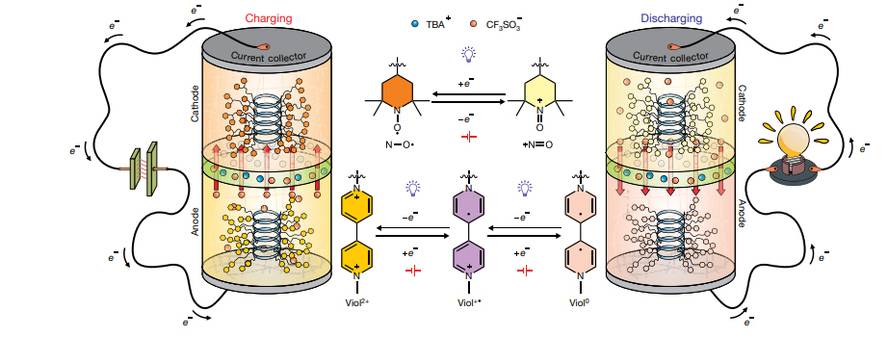
The development of lithium-ion batteries has had direct consequences in the technological market, with a significant increase in portable devices and electric vehicles. However, the rapid growth of these batteries has shown that extraction of lithium, cobalt and other limited mineral resources can cause serious environmental problems and is therefore unethical. On the other hand, only a small percentage of lithium-ion batteries is recycled. Several chemists at the University of Texas A&M propose to develop more ethical batteries: generation of organic batteries without metals. And they have already created the first prototype.
These innovative batteries have replaced the use of metals with polypeptide chains. In these chains, biologists and nitroxide radicals that function as anode and cathode respectively have joined together as active groups. These batteries have reached a maximum load capacity of 37.8 mA h g-1.
Polypeptide chains based on glutamic acid have been created by adding groups of chlorine or alquinyl to have redox activity. The researchers have stated that active organic materials have managed to remain stable during battery operation and that they can be subsequently degraded by acid conditions: polypeptide chains are retransformed into amino acids along with other degradation products. It seems, therefore, that they can be recyclable and cause less safety problems than lithium batteries.
This is a first step in the development of organic batteries, with great technological challenges for the future, including the elimination of the dissolution of the active material and the increase in battery capacity.





