Mercury from fascination with terror

The most widespread use in antiquity is that of a mineral, the cinnabar, not that of metallic mercury. It should be noted that pure mercury (liquid metal) is present in nature in small quantities; its minerals are much more abundant, especially sulphur mercury, cinnabar.
Cinnabar is a bright red mineral whose dust was highly appreciated as cosmetic in Ancient Rome. It was used to make creams and touches, to make medications, and especially to redden the cheeks. The bermilon used to represent one of the red colors is, in short, cinnabar dust.
Therefore, the Romans needed the cinnabar, sold it and exploited the mines of cimabrio. The largest of these mines was undoubtedly that of Almadena, in Ciudad Real.
Major mine

The Antiguo Plinio is one of the oldest quotations of this mining complex. According to Pliny, at least, a. C. C. IV. This mine was exploited since the nineteenth century, "the best bermilon of the empire". Civilizations that dominated the environment in the following centuries also took advantage of the mine. For example, the Arabs made centuries there (from VII to XIII), and their reflection has remained in the name of the mine, which originally was Hins Al-Maden, the mine.
The first farms were not very large. In Almadén were the best times; the greatest blooming of the mercury mines came after the conquest of America. XVI. In the mid-twentieth century, a novel method of silver extraction of the mineral was invented: amalgam with mercury. This led to a sharp increase in demand for mercury, which required a large amount of mercury for the rich silver mines that exploded in America, which revolutionized the market. What until then is only sold in small quantities became a huge business.
In mining they were able to take advantage of the virtues of mercury and, although today it is hardly used to extract silver, it is used for gold. One of the most characteristic aspects of mercury is its ability to create amalgams with different metals. Mercury forms potent amalgams such as gold, silver, zinc, lead, and tin. However, with iron it does not generate amalgams, so for centuries iron containers have been used to pack the fugaz liquid of mercury for transport.
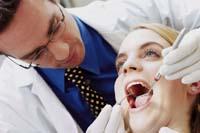
Amalgam has an infinity of uses. In the dentist's office, for example, they are used daily; the holes of the teeth and teeth fed by caries are covered with an amalgam. The amalgam of filling is composed of several metals, mainly silver, copper and tin. In this way a very appropriate material is obtained: it lasts a lot, has a hardness similar to the tooth, good behavior against cold and heat...

Moreover, mercury currently has other uses. It is used for the manufacture of batteries, high precision measuring devices, switches, fluorescent lamps, etc. In the electrochemical industry, much has been used to extract sodium from fat, since mercury forms amalgam with sodium. For the manufacture of bleach, for example, an electrolysis is performed from the marine water or the serum, making a current pass between two electrodes. In one electrode chlorine is released and in the other, of mercury, sodium is dissolved, an amalgam is formed and from this amalgam it is possible to obtain sodium metal or produce bleach.
Seeds, fish and tragedy
Mercury is also used as fungicide for the conservation of seeds, not as mercury, but as mercury compounds. Unfortunately, this use was well known in 1972 and 1973. In those years, more than six thousand people were poisoned in Iraq and five hundred died. They were poisoned by consuming wheat treated with inorganic mercury. In fact, they had a severe famine and sent them wheat from several European countries. It was to plant wheat, not for direct consumption, but it seems that it was late to sow, and pressed by hunger they ground the wheat and made bread.

It must be said that behind the bad name of mercury there is, to a large extent, an organic compound of mercury: methyl mercury. Mercury is much more toxic than mercury, and the most serious poisonings are therefore. Another case of famous poisoning is that of the bay of Minamata, on a Japanese island. A company poured into the river for years as a catalyst
The mercury of methyl he used was accumulating in fish and seafood. As a result, thousands of people who fed them became ill (including animals). It affected people of all ages, with lack of coordination, loss of sensitivity in hands and legs, vision and hearing, causing paralysis and death in the most serious cases. The first appearance of the disease took place in 1956, and since then there have been several. Due to these facts, methylmercury poisoning is known as Minamata disease.
After such tragedies, it is no wonder that mercury sounds a terror. Numerous studies have been conducted to measure the risk of mercury. In general, the questions are more numerous than the answers, but some eye-catching data have been presented. For example, the main source of mercury of many people is pastes. Breathing consists of the taking of mercury vapour, but to date it has not been shown that this can harm, although this vapour can be toxic (they knew perfectly the miners of mercury), since most of the inhalation is expelled.
Another main source of mercury is the diet. Since mercury is found in all the media (air, soil and especially water), almost all foods have some trace of mercury. Some fish, for example, accumulate a lot of mercury in the body, as evidenced by the success of Minamata. And the worst is that they have the most in the form of methyl mercury. The mercury of methyl expands easily in the tissues of living beings, giving rise to bioaccumulation and biomagnification, that is, it accumulates to the life of fish, increasing the amount of mercury as the trophic chain is ascended. For this reason, fish with more mercury are those found at the last end of the trophic chain, such as swordfish, some sharks, tuna...
However, one cannot say what percentage of mercury is methylmercury of each piece of fish, since studies show that it varies a lot from one grain to another of the same species. Therefore, it is assumed that all the average mercury of each species of fish is, in case, methyl mercury. Therefore, and because the specific analysis for methyl mercury is more expensive.
Accursed Mercury
As seen, the real risk for the health of the majority of the population is methyl mercury and not mercury. And yet, in general, mercury is spoken of. But it is not surprising, since mercury is transformed and the final product is usually methyl mercury.To begin with, it must be said that mercury easily expands from place to place, since mercury vapor lasts years in the air. This mercury vapour is derived from human activities (burning of coal, incinerators, etc. ), but to a large extent also by natural processes (volcanic eruptions or meteorization of rocks). The truth is that, once in the middle, mercury changes form and other mercury compounds are generated. For example, part of the mercury vapor becomes an inorganic compound that is deposited in soil or water. There, microorganisms make it methyl mercury. Therefore, even though metallic mercury flows into the middle, there is methyl mercury in the majority of the earth and water masses.
Therefore, the situation is worrying. But, unfortunately, mercury remains a rather unknown subject. The United Nations Environment Programme itself recognizes that there is not enough data and that much research needs to be done. However, they highlight that the decisions to reduce mercury emissions cannot be delayed and ask the international to take measures such as, on the one hand, the reduction of the activity of the mercury mines and the use of raw materials containing mercury, on the other, the use of products and processes alternative to mercury, on the other hand, the control of mercury emissions and, on the other, the correct management of the residues containing mercury.

Return to Almadena
The European prohibitions have influenced, of course, in Almadén. In 2001 the extraction of mercury in Almadén was abandoned, continuing to work for a couple of years with the opening of the warehouses, so in 2003 it stopped working definitively in the mine of Almadén.
The world's largest mercury mine is therefore closed, and not by the absence of cinnabar in its bowels, but by the European Union's prohibition of extracting mercury. The geologist Ángel Hernández, who worked for 29 years in Almadén, is very sorry for this. According to Hernández, they complied with the most demanding security measures, both in terms of workers' health and environmental matters. Proof of this is the epidemiological study carried out by the EPA (Environmental Protection Agency) of the United States about fifteen years ago, which found nothing relevant neither in the neighbors of the region nor in the vegetation and fauna.

Now they begin a new era in Almadén. It has been prepared for visitors, who have dedicated the area, opened a museum of miners and a mining park in which you can visit the interior of the mine. In Almadén, mercury is today a story, but in the world the historical book of mercury is still open.









