Supercapacitors: energy accumulators of the future
Energia Metatzeko Sistemen ikerketa-taldea, Mondragon Goi Eskola Politeknikoa, Mondragon Unibertsitatea
Energia Metatzeko Sistemen ikerketa-taldea, Mondragon Goi Eskola Politeknikoa, Mondragon Unibertsitatea
As seen at the Paris climate summit, almost no one denies that there is global warming. This shows that concern for the environment is growing steadily in our society. Unfortunately, the wild exploitation of the world's limited natural resources continues. According to experts, in this decade we will overcome the peak of oil if we have not already overcome it. That is to say, at this moment the world extraction of oil is around its maximum [1], which from now on will be decreasing. Therefore, if we do not want a dark future for the coming generations, we need to change the energy model. Clean energy sources should replace fossil fuels.
Thus, in the future, the use of electricity will increase apart from fossil fuels. This change will have advantages not only from the environmental point of view but also from costs, safety and efficiency. Therefore, there are important changes in the energy model (production-distribution). Unfortunately there is a problem. Although electricity generation is relatively simple, it is not so easy to store it efficiently. The truth is that in recent years, despite advances in batteries and other energy accumulators, they have not been enough to cover the needs that come to us. We need new accumulators, cleaner and more powerful.
Supercapacitors
In this context, it is worth mentioning the supercapacitors used for a long time (“In technical language the supercapacitors in English are known as Electrochemical Double Layer Capacitor, EDLC. However, in terminology there is great confusion, since they are called ultracpacitors, electrochemical capacitors, boostcaps, goldcaps, etc.”) the development experienced in recent years. In fact, so far, supercapacitors have been used primarily in high-power applications. However, other applications can be of great interest for their technical advantages [2]: high power density, fast loading and unloading processes, usability in a wide range of temperatures, very long working life and without maintenance.
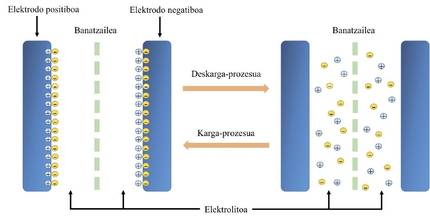
Supercapacitors, like batteries, accumulate and transfer loads in the processes of storage and energy release. In the case of batteries, chemical reactions are produced in the loading and unloading processes. Unfortunately, the chemical processes are not completely reversible and with time the batteries are degrading and shortening their life. On the contrary, the loading and unloading processes of the supercapacitors are due to a physical effect. In the electro-electrolyte interface there are two distributions of opposite sign load (see figure 1), called double layer (double layer). The German scientist Hermann von Helmholtz first described the double layer capacity in 1853. This effect is reversible and occurs much faster than chemical reactions. Therefore, the supercapacitors can be loaded and downloaded quickly. In addition, they do not degrade, so their work life is very long.
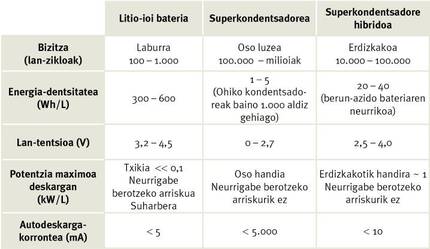
Unfortunately, compared to batteries, the energy density of the supercapacitors is low. That is, they accumulate less load for the same weight as the accumulator (see Table 1). In fact, the accumulation of energy in the supercapacitors is proportional to their capacity, which is proportional to the surface of the electrodes [3]. Specifically, the loads are added in surface. Therefore, to greater surface, greater capacity of accumulation of loads. Therefore, to increase the energy density of the supercapacitors, it is necessary to increase the surface of the electrodes. In addition, the surface area of the electrodes also limits the capacity of electric current transport, thus limiting the maximum power that the supercapacitor can provide. Therefore, the surface increase has another advantage, the increase in power.
Nanomaterials
In recent years, nanomaterials are being used to increase the energy density of supercapacitors. The microscopic structure of nanomaterials increases the surface of the electrodes, allowing supercapacitors to accumulate more load. Activated carbon has been used in the two electrodes of previous supercapacitors. Activated carbon is a amorphous material, very porous. Instead, nanomaterials [4] have been used as carbon nanotubes, graphene based electrodes, carbon from carbides (Carbide-Derived Carbon, CDC), etc.
Carbon nanotubes are formed by carbon atoms. Carbon atoms form hexagons due to their relationships. Thus, in the mural carbon nanotubes, the hexagons of the carbon atoms form the surface of the tube and the tubes of a diameter nanometer are obtained. The vertical alignment of these nanotubes significantly improves the physical properties of the electrode with respect to activated carbon. For example, it is possible to increase the surface of the electrodes.
Graphene has a structure similar to carbon nanotubes, but it is a flat layer of carbon atoms. In graphene, carbon atoms are united in a plane as a net of honeycomb. The main problem of the use of carbon and graphene nanotubes is its high price due to the complexity of its manufacture.
Hybrid supercapacitors
The needs of society make researchers look for new materials for electrodes. As we have seen, one of the options is to use nanomaterials, but it is not the only one. A conventional activated carbon electrode can be replaced by a battery electrode. That is, it has begun to use a conventional electrode used in supercapacitors and another electrode used in batteries to create a new accumulator. This type of accumulators are called hybrid supercapacitors or asymmetric supercapacitors [5].
Scientists are using various chemicals to create the electrode of hybrid supercapacitor batteries [6]. With them, a higher energy density of hybrid supercapacitors has been achieved, which allows to build smaller accumulators. Among the chemical compositions obtained to date stand out the nanoporous hydroxide nickel and the active carbon; the graphene by laser drawing obtained from the graphite layers (Laser-Scribing Graphene, LSG) and manganese dioxide; the porous nanooxide of metal doped from the technology of the liquid crystal mold; and the lithium oxide, doped material. This last chemistry is the most widespread in the market and is known by experts as the capacitor lithium ion (LIC).
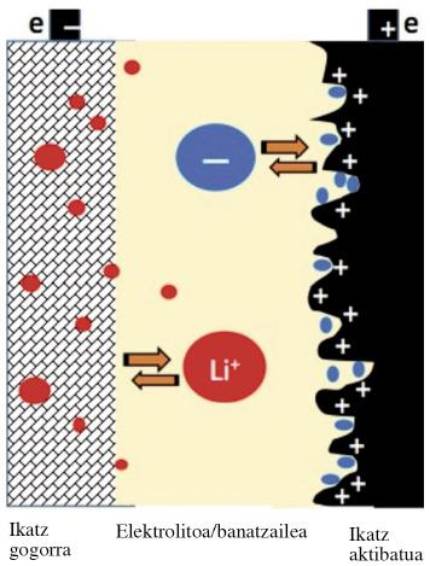
In the hybrid LIC supercapacitors, one of the electrodes is like a conventional activated carbon supercapacitor. This electrode is a cathode (positive electrode) that captures and releases the ions from the electrolyte in the loading and unloading processes (see figure 2). The other electrode is the anode (negative electrode), which is an electrode as a lithium ion battery, formed by hard carbon pre-doped by lithium. In this electrode the lithium ions of the electrolyte are absorbed and released in the loading and unloading processes. The use of the lithified anode increases the energy density by increasing the working voltage of the accumulator. However, the loading and unloading characteristics of the accumulator are similar to those of a conventional supercapacitor. However, the energy density of LIC hybrid supercapacitors is 5-7 times higher than that of a conventional supercapacitor. This is a great advantage as it allows to use smaller accumulators for any use.
On the other hand, the LIC supercapacitors do not present problems in lithium ion batteries. For example, when lithium metal deposits are not produced in the loading and unloading processes of LICs, no dendrites are generated, so there is no risk of creating short circuits. The hybrid supercapacitor behaves as a conventional capacitor in the loading and unloading processes. In fact, the electrolyte ions move in the electric field produced by the electrodes, inserting and extracting them, without redox reactions in the batteries. This is evident in the behavior of the supercapacitor voltage. In the charging and discharge processes of a battery the voltage varies very little, while the voltage of a supercapacitor increases during the charging process and decreases during discharge. In addition, LICs can contain large amps and stresses without danger. This will prevent the application of lithium metal in the electrodes. In the fast charging and discharge processes of lithium ion batteries, lithium metal is placed inside due to redox reactions. This can provoke unbridled heating and an increase in tension within the cell, and can occur in extreme cases fires and explosions. Therefore, supercapacitors avoid the risks of lithium-ion batteries.
Form factor and future
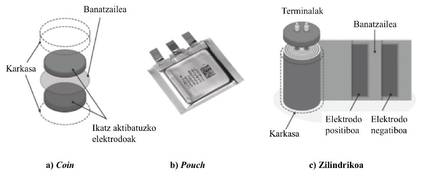
The typical form of supercapacitors on the market is cylindrical (see figure 3) or like a coin (coin cell). This can be a problem because, in short, the supercapacitors in this way cannot be used in many applications. Therefore, manufacturers have begun to commercialize flat supercapacitors to introduce them slowly in the applications that use the batteries. They are of type pouch or prismatic. The shape and dimensions of energy accumulators are very important for their deployment in applications, despite their small impact. With a small flat shape, new supercapacitors can be used in areas where current supercapacitors cannot be used. In addition, this has the advantage that flat cells can be manufactured in battery production lines, thus reducing production costs. Finally, flat cells can be packaged better, which increases energy density and reduces costs. Since supercapacitors are not heated more than enough, they can be packaged much more efficiently than lithium-ion batteries. In short, they do not need cooling or electronic batteries management (Battery Management System, BMS).
On the other hand, in a supercapacitor, most of the price corresponds to the components, being the electrodes the most expensive among them. However, all the innovations that are being produced are causing the supercapacitors to be increasingly being used. And, of course, as production increases, the prices of accumulators decrease. With the drop in prices, its scope of use is significantly expanded to extend in areas until now unthinkable.
Finally, if the energy density and the size/cost ratio are improved, the application option of these innovative supercapacitors will be wider than ever, facilitating the leap to cleaner energy. Smaller supercapacitors can feed small electronic devices, as well as overload devices. But the aim of this article is not to talk about the fields of application of supercapacitors. We leave it for another.