Developing sustainable polymers for food packaging
The United Nations has published several sustainable development goals. These include reducing hunger, maintaining food security and improving food. To address these challenges there are several options, including lengthening the survival of food with proper packaging.
However, the XXI. Plastics used for packaging in the 18th century have created a serious problem due to poor waste management. The objective of this doctoral thesis has been the need to develop new environmentally sustainable materials to solve this problem.
Packaging for food waste reduction
According to studies by the Food and Agriculture Organization of the United Nations (FAO), 14% of the food produced in the world is damaged before reaching the store. According to FAO, in 2019, more than 690 million people were hungry. Therefore, it is essential to reduce the generation of food waste, in this challenge it is urgent to use suitable packaging. Packaging guarantees the quality and safety of food during transport, storage and consumption of the product. Keep in mind that food can be damaged by physical, chemical or biological changes.
Packaging uses materials such as metal, glass, cardboard and plastic (Figure 1). Each type of material has its advantages and drawbacks. For example, metal and glass are waterproof, i.e., external gases or vapors do not pass through the container and are easily recycled. However, they have some drawbacks: on the one hand, a lot of energy is spent to process these materials, and on the other, they are very heavy, which affects transport, since more fuel is consumed.
Plastics, however, are cheap, easy to process and lightweight. However, in recent years the inadequate management of plastic waste has generated a serious environmental problem. There are currently three ways to manage plastic waste: landfill deposit, mechanical recycling (the physical properties of the material resulting from this process are worse) and incineration in incinerators. All these alternatives present a number of disadvantages that are unsustainable with the environment. Solutions such as the use of biodegradable or the use of chemically recyclable polymers have been proposed to address this problem.
Polymers: packaging materials
The polymeric materials used in packaging must have certain characteristics: they must be transparent, have good barrier properties and have good mechanical properties.
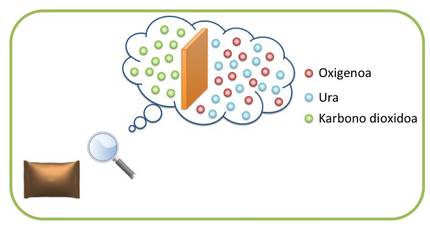
Before explaining what good barrier characteristics are, you have to understand what permeability is. Polymers, unlike glass and metal, are permeable to various gases. That is, small molecules can cross the polymer, so the transport of gas molecules occurs from the inside of the container to the environment and vice versa (Figure 2). The transport of this gas or steam can damage the packaged product. On the other hand, the aromatic compounds of food can be lost, reducing their quality. Therefore, it is very important to know the barrier characteristics of the polymers to be used in packaging. In general, a material is considered to have good barrier characteristics when its permeability to gases and vapors is low.
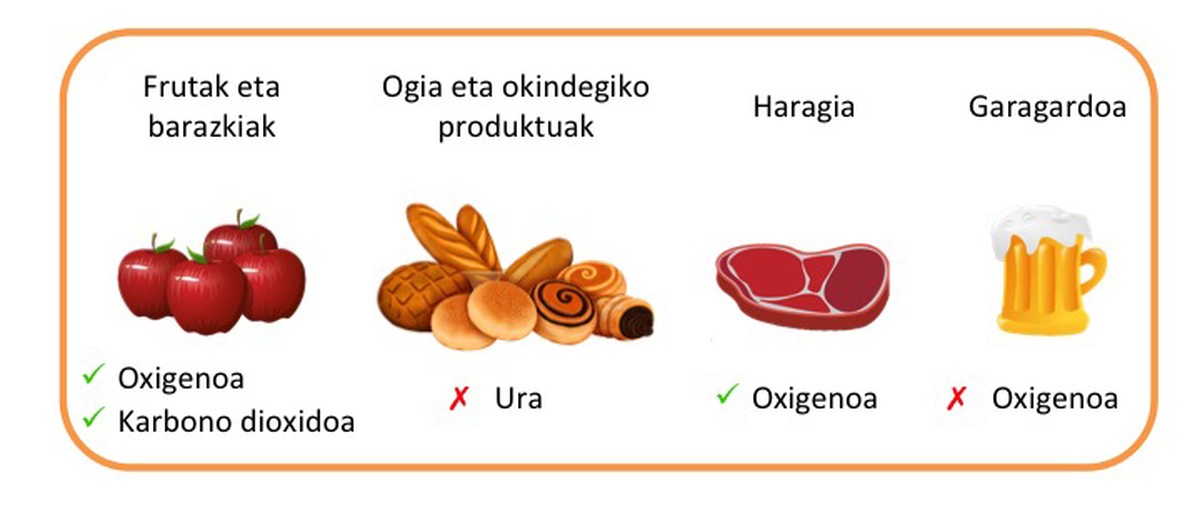
The characteristics of the product to be packaged will be taken into account for the selection of the packaging material (Figure 3). For example, fruits and vegetables breathe, so the material used in packaging must be permeable to oxygen and carbon dioxide. In the case of red meat, the material must be permeable to oxygen, otherwise the meat will lose its red color, acquiring a brownish color. In products such as bread, water must be avoided to maintain a crunchy texture. The material used for the packaging of beer should avoid the entry of oxygen by altering the organoleptic properties of the product. Therefore, as indicated, materials must have properties suitable to each food. However, in general, the main challenge of the packaging area is obtaining low permeability materials to gases and vapors.
Currently, the most widely used materials in packaging are polyethylene, polypropylene and polyethylene terephthalate, for their low cost and for their physical properties. However, these types of materials are not biodegradable, but once used they accumulate in landfills or in the environment, increasing the problem of plastic waste.
Biodegradable polymers: plastic waste solution?
A solution to this problem can be the use of biodegradable polymers, since under certain conditions they degrade by obtaining substances such as biomass, carbon dioxide or water. This prevents waste generation and promotes circular economy.
The most commonly used biodegradable polymers are polylactide and polyaprolactone, but they present some drawbacks: in general, the mechanical properties are inadequate for their fragility and on the other hand, they have a high permeability to gases and vapors. There are several options to improve the characteristics of these materials, one of them being the mixture with polymers with better properties. This allows to improve the properties of biodegradable polymers in an economical and simple way. This mixture can be miscible or immiscible. Normally polymers are immiscible, that is, they are divided into two phases, just as oil and water do not mix. The goal is to achieve a miscible mixture, since this type of mixes have better physical properties.
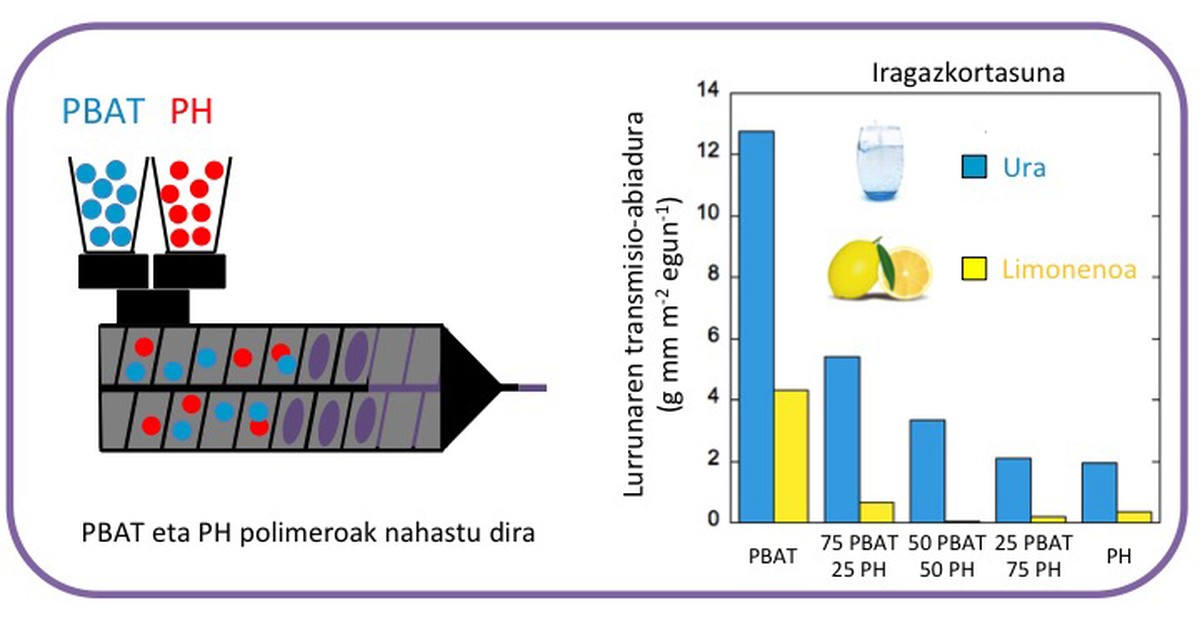
In this thesis the biodegradable polymer poly(adipate-co-terephthalate butyl) (PBAT) has been studied for its mechanical properties suitable for use in packaging. It has a high permeability to gases and vapors in terms of its barrier characteristics. Therefore, to improve the properties of PBAT it has been mixed with the phenoxi resin (PH), which has a low permeability (Figure 4). The mixtures obtained are miscible in the wide range of composition analyzed. In terms of barrier characteristics, a notable improvement is observed, since only the addition of 25% phenoxi resin significantly reduces the permeability to water vapor and limonene. On the other hand, the degradation of these mixtures has been studied to study the influence of the addition of phenoxia on degradation. While mixing degradation is slower than in PBAT, its degradation rate is similar to polylactide or polyaprolactone. Therefore, it can be concluded that the mixtures obtained are suitable for use in packaging.
Chemically recyclable polymers
In recent years, chemically recyclable polymers have aroused great interest. These polymers, once used, can be recycled by recovering initial compounds. The chemical recycling and mechanical recycling mentioned above are totally different. In mechanical recycling plastic waste is crushed and sorted according to the type of polymer. Then each type of polymer is heated and shaped to get a new piece. Due to the reprocessing of the material, the properties deteriorate, so this procedure cannot be repeated as many times as desired (Figure 5).
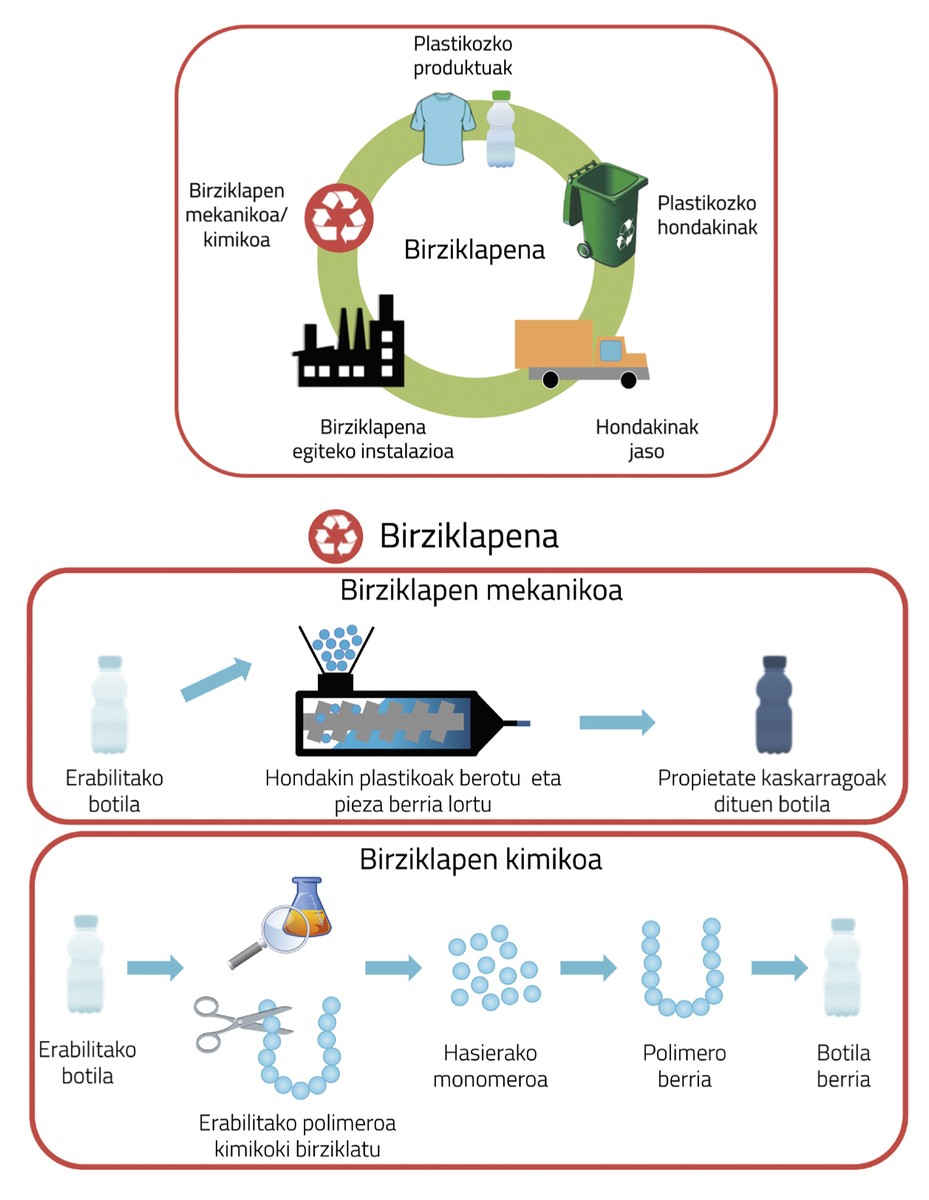
On the other hand, when it comes to chemical recycling, to understand the process it is necessary to know first how polymers are formed. As can be seen in figure 5 (see chemical recycling section), polymers are long chains formed by repetitive units. For example, if the repetitive unit is a pearl, the polymer chain is the necklace that forms joining one pearl with the other. Once the polymer is used, the initial pearls can be chemically recycled and the necklace can be reformed. Therefore, these materials are infinitely recyclable and their physical properties are not lost.
Chemically recyclable polymers have some problems: on the one hand, it is very difficult to obtain a totally recyclable material and, on the other, if it is easily recycled, the physical properties of the material are deficient, for example, they do not have mechanical properties suitable for use in packaging. For this reason, polymers of this type that have been studied so far cannot replace those currently used in the market.
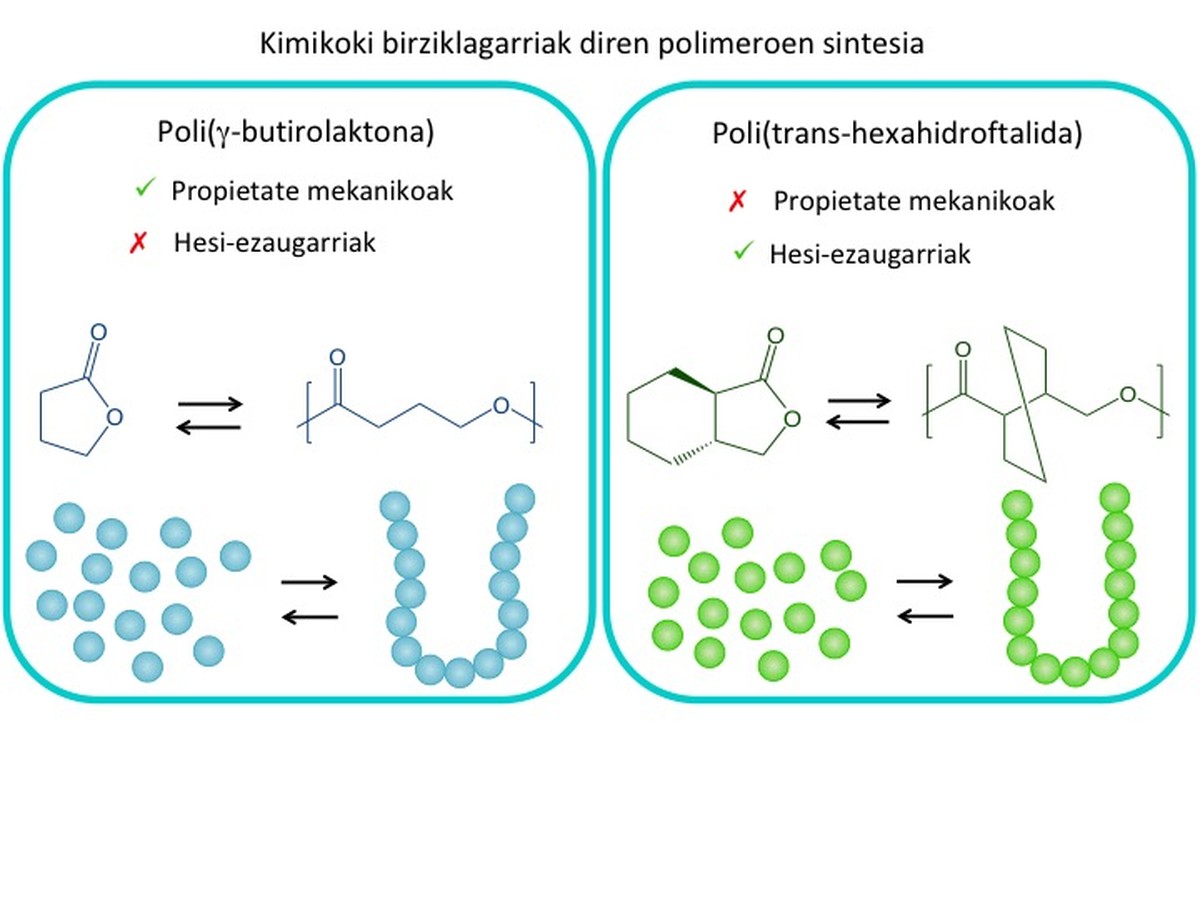
This thesis has been used to synthesize polymers based on butyrolactone for use in food packaging. As shown in Figure 6, the <br class="xliff-newline" <br class="xliff-newline" /> /> In addition, a carbon ring is attached to an oxygen atom. This five-atom structure contributes to the chemical recycling of the polymer. Poly (>-butyrolactone) has excellent mechanical properties, but its permeability to gases and vapors is high, so it is not suitable for use in packaging (Figure 6). On the other hand, there has been <br class="xliff-newline" /> a transformation of the <br class="xliff-newline" /> This polymer is also chemically recyclable, but in this case it has opposite properties: its permeability to gases and vapors is very low, which is an advantage for packaging, but its mechanical properties are inadequate for its fragility (Figure 6).
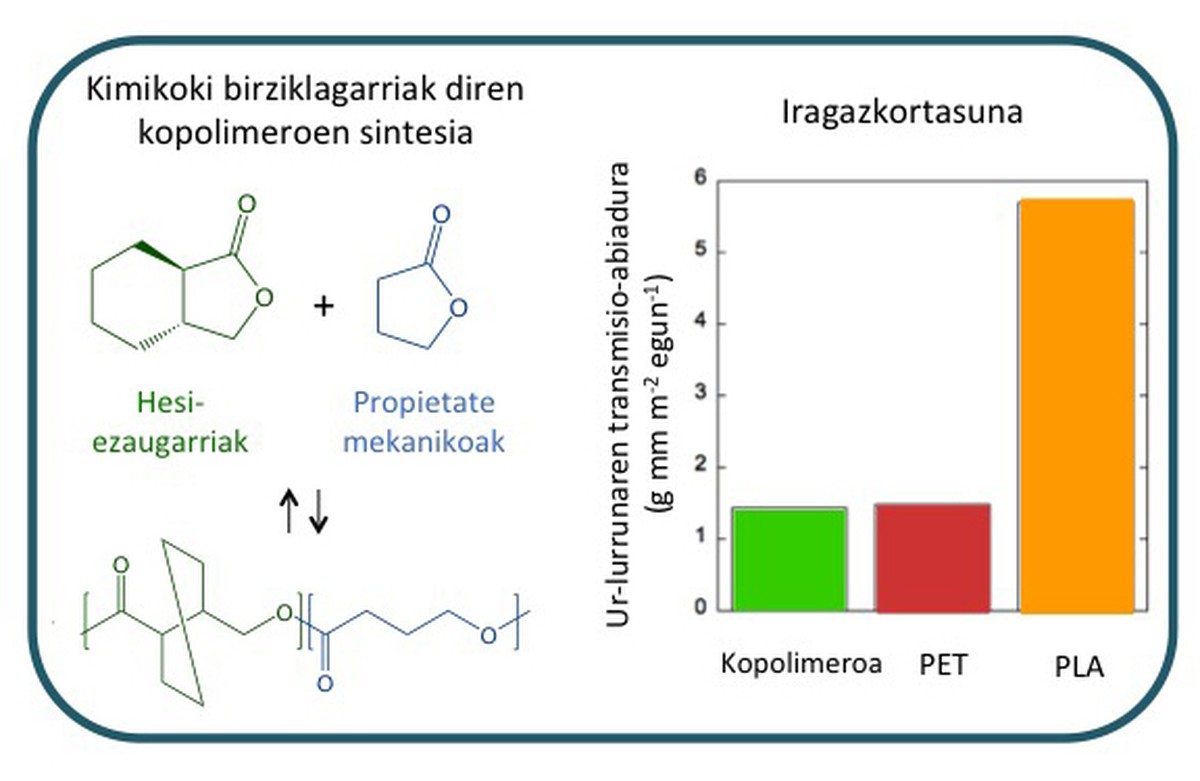
Therefore, both monomers have been combined to obtain copolymers (Figure 7). As can be seen in the figure, the permeability of the water vapor copolymer is much lower than that of polyethylene terephthalate (PET) and polyethylene (PLA). The mechanical properties of copolymer are better than those of polyethylene terephthalate and polyethylene, which is fragile. Consequently, these new materials can replace the polymers currently used in the market.
Bibliography
FAO, IFAD, UNICEF, WFP and WHO, The state of food security and nutrition in the world 2020. FAO, Rome, 2020.
J.V. Koros, Barrier Polymers and Structures. American Chemical Society, Washington, 1990.
J. Areizaga, M. Cortázar, J.M. Elorza, J.J. • Synthesis, Madrid, 2002.
Free announcement Gross, B. Kalra, Biodegradable polymers for the environment, Green Chemistry, 297, 803–807, 2002.
V. Syracuse, P. Rocculi, S. Romani, M.D. Rosa, Biodegradable polymers for food packaging: a review, Trends Food Science and Technology, 19, 634-643, 2008.
X. Tang, E.Y.X. Chen, Toward infinitely recyclable plastics derived from renewable cyclic esters. Chem, 5, 284-312, 2019.
G.W. Coates, Y.D.Y.L. Getzler, Chemical recycling to monomer for an ideal, circular polymer economy, Nature Reviews Materials, 5, 501-516, 2020






