Death of molecules
XVIII. Late nineteenth century Throughout the twentieth century there was a great revolution in chemistry. They belong to this time, for example, the founders of modern chemistry Lavoisier, the law of conservation of the mass enunciated, or the atomic theory of Dalton. Logically, the new theories based on experimentation supposed the exclusion of those that until then were accepted. Perhaps the best known example is the XVII. Scientists of the end of the century --J. J. Becher and G. Stahl is the decline of the theory of phlogisto proposed to explain combustion. The communication of advances in different areas of research led to close relationships between scientists. Academies, scientific journals and meetings were spread everywhere. In most cases, the exchange of ideas was the origin of enthusiastic discussions or scientific conflicts, but sometimes they were a source of reconciliation and scientific collaborations. In the latter case we can situate the controversy that Friedrich Wöhler and Justus von Liebig had on the isomer of chemical compounds as a starting point of numerous collaborations.
However, the elimination of obsolete scientific ideas was an important leitmotiv of many scientists, and the work done with this intention led to great advances in science. Therefore, we must not forget that all theories, even the erroneous ones, have fulfilled their function in the history of science and, of course, of chemistry. While the above mentioned facts - phlogisto or isomeria - are really attractive, what I will bring to the square is another of those beautiful stories of the history of chemistry: F. F. When Wöhler synthesized the urea and its consequences in the Bitalist theory.
Vitalismo vitalismo
Vitalismo XIX. At the beginning of the twentieth century it was a thought in pure state. The basic principle of vitalist theory was that between the organic and inorganic world there is an impenetrable abyss. For the French vitalist physiologist Xavier Bichat, the living bodies survived the ability to confront inorganic forces that tended to be destroyed. Life itself was a competition between the opposing inorganic destructive forces and the force of life. To maintain life, it was necessary for vital forces to succeed in the face of chemical forces. The aim of the chemical forces was to convert living organisms into basic chemical elements. According to this vitalism approach, organic and inorganic chemistry were regulated by absolutely opposing forces.
Another important example of vitalist theory is the book A Manual of Chemistry, written by John Webster, which was used at Harvard University before the Wöhler experiment. According to Webster's explanations, the organic bodies were formed by four inorganic elements, but life was more than the union of these four elements: there was a principle of life independent of the chemical forces. Consequently, the chemical world was dominated by two forces: chemical affinity and life force. On the one hand, chemical affinity served to explain the inorganic reactions and, in addition, it was in the hands of the chemicals to dominate and use that force. On the other hand, the life force regulated the organic world, justifying one's own life. This strength was not -- and never depended on the chemicals of the wear-. Thus, vitalism indicated that it was impossible to synthesize organic compounds in the laboratory. The force of life was not the tool of science.
Synthesis of urea
In 1828 Wöhler sought a reaction to synthesize amonic isocianate (NH 4 NCO) due to research on isomería. To this end, it heats the solution containing silver isocianate (AgNCO) and ammonium chloride (NH 4 Cl), considering that the following chemical reaction would occur:
GaNCO + NH 4 ClNH 4 NCO + AgCl
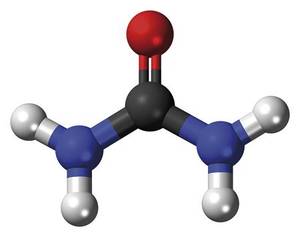
However, the crystals obtained as a product of the reaction corresponded to a compound that Wöhler did not expect. Aware of this, he analyzed and analyzed the white substance in various ways and concluded that it should be urea. Urea, CO(NH 2 ) , is a chemical compound known since 1773 that reaches urine as a result of protein metabolism. But how was urea obtained? In fact, the ammonium isocianate - from whom Wöhler was looking - was transformed into nothing else born from the cause of the isomerization reaction giving urea:
Standard NH 4 NCO
The synthesis of urea was not very interesting, but Wöhler's experiment showed for the first time that it was possible to synthesize a biological molecule without the need for life force. In other words, it synthesized an organic molecule from only inorganic components. From the current point of view, although we can consider the synthesis of urea as the starting point of a new scientific field -- organic chemistry -- XIX. Many scientists of the early twentieth century did not see the end of vitalism so clear.
Revision of the myth of Wöhler
Wöhler's own vision of the experiment has been the subject of multiple discussions and various interpretations of the synthesis of urea: that he was a fervent rival of the vitalist theory, that he remained vitalist or that he had not realized the importance of the experiment. In one way or another, faced with the incorrect interpretations of many authors, the myth of Wöhler was underway. A myth that has come so far. In this sense, in 1996 P. S. S. Cohen and S. M. M. Cohen analyzed 35 textbooks of chemistry and came to worrying conclusions: in most books, the event about Wöhler appears with bad historical nuances, claiming that on many occasions the synthesis of urea discarded vitalism and organic chemistry arose. In addition, the importance of the experiment is manifested in a confused way in most textbooks, according to research.
According to historical revisions of the event, despite the widespread conviction among the chemists, it is an unacceptable historical error to think that the synthesis of urea caused the failure of vitalism or that Wöhler's goal was to nullify vitalist theory. In his article of communication to the scientific community of the synthesis of urea, Wöhler once again refers to the relationship between his experiment and his vitalism. It gives more importance to the isomerization reaction obtained and J. J. From the letters he wrote to Berzelius, a renowned chemist and former Wöhler professor, it follows that he did not believe that he would cross the border between the organic and inorganic world. After the discovery, Wöhler continued to believe in the vital force of molecules. The vitalist theory was so rooted that among the scientists of the time the synthesis of urea was not enough to break those ideas. But, as a result, what is the importance of the experiment then, if the one who did so did not consider it important? According to vitalists, it was impossible to synthesize an organic compound in the laboratory. But when Wöhler did, why didn't scientific thinking change?
A step closer
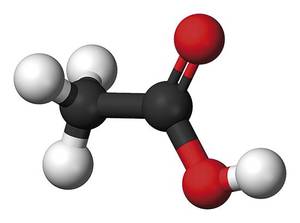
Although the failure of Bitalism did not come suddenly, some of Wöhler's contemporaries changed their vision of the life force. Subsequently, those who did not agree with vitalism, starting from the synthesis of urea, synthesized other organic molecules in the laboratory: Kolbe, acetic acid; Berthelot, acetylene, benzene or methane - a very extensive list of the latter -; Strecker, several amino acids, etc. Thus, little by little, those who bet on vitalist theories decreased, and there was a notable decline after the synthesis of acetic acid Kolbek (1845). Experimentation clearly showed that organic molecules, like urea, were dead.
Between 1844 and 1870, the number of known organic compounds went from 720 to 10,700 -- that of the inorganics from 3,250 to 5,300.- It was, therefore, a very prosperous time in organic chemistry. As they were accumulating and accumulating arguments against vitalism, vitalism became obsolete in the twentieth century. For the early twentieth century. Although Wöhler's urea synthesis was nothing more than the first step of a long road, it was an essential step. In this is the importance of the synthesis of urea, which provoked a debate on the force of life and vitalism, initiating one of the most prosperous stages of organic chemistry.
Challenges of the future
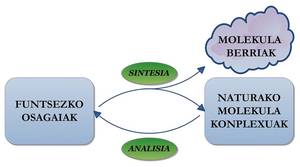
The synthesis of molecules mentioned above, together with many others that have been made later, demonstrate the ability of chemicals to produce compounds present in nature or non-existent molecules. By studying the complex substances present in the nature and understanding of the reactivity and properties of the molecules, scientists each day obtain new molecules that can have really interesting characteristics. The synergy between analytical and organic chemistry offers wide possibilities --unlimited, agi-- in the field of molecular synthesis.
Finally, it should be remembered that, like synthetic organic chemistry, synthetic biology is also a leading science. It should be noted, for example, that in 2010 biologist Craig Venter achieved a cell with synthetic genome when he released it in the journal Science. Like synthetic organic chemistry, synthetic biology will answer many questions in the coming years and propose more challenges for the future. And is that science has made it clear that organic molecules have no life force, but can one get life itself in the laboratory? Will we soon meet another Wöhler or Kolbe?
Bibliography Bibliography Bibliography
Thanks to the Department of Education, Linguistic Policy and Culture of the Basque Government for its collaboration with the predoctoral grant.