Methane: Source of energy?
The activation of the C-H link of methane at industrial level, in agile reaction conditions, is one of the most important challenges of current chemistry. In fact, if this activation were possible, methane would be one of the most used chemical compounds, not only in the production of energy, but in the obtaining of molecular hydrogen and in the synthesis of more valuable chemical compounds such as alcohols or larger hydrocarbons.
In general, when we talk about activation of saturated atoms, formed by C-C and C-H linkages, we are talking about systematically breaking one of your C-C or C-H links. This is the first obstacle to overcome in many of the uses of these compounds. These processes, however, are highly expensive from the energy point of view in conventional reaction conditions, limiting the current use of saturated reaches to energy production by combustion mainly. This use exploits the energy capacity of these compounds, but not the possibility of obtaining more useful and valuable compounds from this compound.
To the extent that methane, CH 4, is the smallest of the saturated reaches, it can be said that its activation has certain particularities. On the one hand, to the extent that the four methane hydrogens are equivalent, their activation does not involve usual selectivity problems in these cases, since the product will be the same. On the other hand, being the strongest C-H links in the reaches of the carbons of the corners, it is clear that methane is an extreme case.
In addition, to understand the importance of activating this compound, the main component of natural gas, it should be taken into account its origin. In fact, methane appears as a product of anaerobic decomposition of organic matter and the metabolism of metanogenic microorganisms, so there are large natural reserves of methane in the world, estimating that the world amount of methane is twice as much as fossil residues.
Much of these natural reserves are concentrated in ocean floors and lakes as methane hydrates. For this reason, methane is often referred to as the gas of lakes. These methane hydrates are methane clatrates formed in low temperature and high pressure conditions, open structures formed by water molecules that host a methane molecule.
It is estimated that more than half of methane and natural gas are in a methane-hydro state globally. Thus, many countries are investigating and studying the possibility of using this methane hydrate as fuel.
In addition to the soils of the oceans and lakes, in the first layers of the earth's crust there are large reserves of methane adsorbed in coal veins. Thus, in many countries this methane is used to reduce dependence on oil. However, the use of this methane also carries risks. This is because it is necessary to use large amounts of water to reduce pressure and release gas from the pores. This, in addition to reducing water levels of aquifers, can cause water pollution.
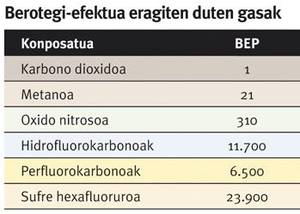
Besides the two risks mentioned, at present there is another one that has acquired a great attention. In fact, the potential greenhouse effect of methane (PIR) is 21 times higher than that of carbon dioxide. Therefore, in the exploitation of the different types of natural methane supplies, it is necessary to take measures to avoid the escape of gases into the atmosphere, which could increase climate change.
In this sense, in many livestock areas, landfills and wastewater treatment plants with high methane production, instead of releasing to the atmosphere the methane generated, is collected and used in energy production. In this way, however, carbon dioxide is generated, the main responsible for climate change despite having a PIR lower than methane. Methane activation can be the key to solving this problem.
The potential greenhouse effect (PEP) reflects the capacity of a gas to cause the greenhouse effect. It is used as a reference carbon dioxide, and the PIR value of a gas indicates the number of times that a certain concentration keeps the heat of the sun in the atmosphere above the same concentration of carbon dioxide.
Looking for the most suitable catalysts
Returning to the activation of methane, to reduce the energy needs associated with the breakdown of its C-H link, catalysts are used in the laboratory. In fact, most of the current research uses transitional metal compounds that appear in the central block of the periodic table. We say compounds because transition metals can be used in different ways: as neutral atoms, as cations, as clusters, or as structures of various metal atoms, or as metal atoms attached to other non-metallic elements. Precisely, on the road towards the activation of industrial methane, the key is to find the most suitable catalyst.
The transition metals usually have other possible structures close to the structure or electronic state of their lowest energy level, that is, to the way to distribute the electrons through the molecule, and when investigating the activation of methane with these catalysts, it is necessary to take into account these situations. This is because, although an electronic situation is at a higher energy level than the other, it is possible that it has sufficient stability and that in this case the reaction occurs.
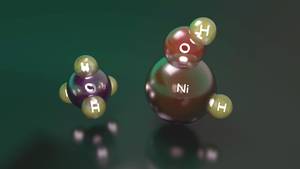
A clear example of this is the reaction between nickel hydride hydroxide cation and methane. In fact, although this compound can activate methane, when it is in its lowest state of energy, it seems a complex between nickel cation and water that does not have the capacity to activate it. On the contrary, when the energy level is higher, the amount of energy that hydrogen needs to move from nickel to oxygen is higher, so it has enough stability to activate methane before providing an inreactive complex between nickel cation and water.
While the reagent of this second state has a higher energy level than that of the first state, it is able to keep it in that electronic state. However, upon reaching an intermediary of the reaction pathway, both situations become interchangeable and, when going from the initial situation to the stable, a complex occurs between the nickel cation and the water, ending the reaction. Therefore, although nickel hydride hydroxide cation is able to activate methane, it is achieved a very low reaction performance, that is, of the reagents that initiate the reaction only a few manage to activate methane.
As we descend in the Periodic Table, the stability of the lowest state of energy increases. In this situation, in the case of platinum which is the largest atom in the nickel group, it can be thought that hydroxide platinum and sulphhydride cations (HP tOH+ and HP tSH+) can react with methane, as it has been shown to be stable enough in their fundamental state.
Taking into account the example of hydroxide hydride nickel cation, and being possible the activation of methane in the lowest energy situation, it is estimated that it increases the probability of obtaining higher reaction yields. In fact, in this case it is not possible to move into a state in which the system presents a lower energy level and does not present sufficient stability to react with methane.
To ensure that these platinum compounds activate methane, this reaction should be investigated by itself, as these two catalysts may not be sufficient to activate methane. Although this study has not yet been carried out, it is known that platinum cation activates methane in mild reaction conditions, and it has been observed that the addition to a transitional metal of groups of hydride (-H), hydroxide (-OH) or sulphhydride (-SH) increases the reactivity of metal with respect to methane. Therefore, these compounds can be suitable for further research.
To finish, we will discard concrete examples and return to the problem of climate change. In reactions between these compound transition metals and methane, molecular hydrogen is often released. Thus, as discussed above, if the activation of methane was possible, methane could be used as a source of H2, and as it is a fuel that does not generate carbon dioxide, this would allow obtaining a "clean" fuel from a compound with a high greenhouse potential.