Oil for almost everything

Of course, you have to keep in mind that there are many types of crude and that not all allow you to get everything. It can be said that practically every deposit has a different type of oil, even nearby.
Types and composition of crudes
Hydrocarbons are organic compounds of carbon and hydrogen and oil crude, including natural gas, is a complex mixture of hydrocarbons. That is, oil is a hydrocarbon.
Different types of hydrocarbons are distinguished according to the existing relationships between carbon and hydrogen atoms of these molecules that form the hydrocarbon mixture: aliphatic when carbon atom chains are open, cyclic when carbon chains are closed and mixed when they present radicals of different characteristics than those of the main chain. The most common crude is paraffinic aliphatic.
But along with hydrocarbons, oil can also contain organic compounds containing other elements: sulfur (the third most abundant component), oxygen, nitrogen or metals (vanadium and nickel are the most abundant). In general, all these components are considered pollutants in the oil refining industry.

Since the composition of oil is so different, the classifications of crude oil are also diverse, depending on what is done. For example:
- Paraffins, unsaturated, naphthenic or aromatic, according to the type of hydrocarbon predominant in the composition.
- API density: arbitrary scale to express crude density and expressed in API degrees. Crude oil is light grade (higher than 38 APIs), medium or heavy (lower than 22 APIs). The more atoms the hydrocarbon molecules contain, the heavier the hydrocarbon. It is the most widely used classification.
- Acids or sweets, if they contain many or few acids and sulfur compounds.
Oil refining
Crude oil can be used directly as an energy source, as fuel in ovens and boilers, but it is not its main use. This is because economic performance is much higher by separating in fractions the components contained in crude oil, which is made in oil refineries. In addition, the burning of crude produces a great pollution by burning or releasing many components that are not separated in the refining and that are not necessary in this combustion.
In any case, both oil and natural gas are pollutants and the main sources of emission are the combustion of fuels, oil refineries and chemical industry.

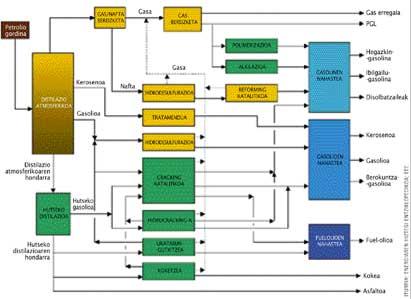
Since oil derivatives began to be used, oil refining has been changing depending on the products intended to be obtained and the technologies available. At first the so-called classical refining became. The physical processes predominated: distillation of crude under atmospheric pressure and separation of fractions, purification of products (usually desulphurisation) and separation of liquefied gases. The only chemical process was the reforming of naphtha to obtain the right gasolines. They are the yellow parts that appear in the refinery diagram.
Conversion processes were then initiated to obtain more specific products. This is because as the consumption structure of petroleum products is modified, intermediate products and, mainly, light products (gasoline and diesel) have increased (appear green in the refinery diagram). Demand for heavy products has declined.
Depending on the characteristics of the crude oil and the final products desired, the structure and configuration of the installation can be modified. However, there are four main objectives in refineries: (i) the distribution of crude oil in fractions; (ii) increasing the value of minimum demand fractions to obtain gasoline and similar products; (iii) increasing the quality of gasoline by reforming; and (iv) cleaning products obtained by final refining. The key processes for this are the same in all refineries (see chart below).
Refineries and the environment
In all these processes carried out in refineries there is a different contamination, although in Europe the ‘relationship’ between refineries and the environment has gradually evolved.
In addition to accidentally emitted gases, oil or other components, the relationship of contaminants emitted in processes is endless: carbon dioxide (CO 2), sulfur dioxide (OS 2), nitrogen oxides (NO x), polycyclic aromatic hydrocarbons (benzene, toluene, ethyl-benzene, volatile organic compounds, carbon dioxide, etc. ), phenul-sulfide, In 1990, for example, 95 European refineries poured 3,340 tons of oil into the water.

According to data from the European Organization of Oil Companies for the Protection of the Environment and Health (CONCAWE), in 1993 one million tons of waste were generated in 89 European refineries. This garbage is divided into three groups: 1) sludge, oil and no, 2) refining residues, liquids, semi-liquids and solids, and 3) waste not from refineries (construction, detergents…). Therefore, they do not take into account the gases emitted into the air. In the table on the right you can see the types of waste they have identified.
The amount of “biological sludge” increases year after year due to increased biological treatment of spills. Catalytic residues have also increased due to increased oil refining performance due to increased environmental obligations of products.
However, sulfur may be of particular importance among the discharges of refineries due to damage caused by acid rain in northern and central Europe. The study conducted by the same entity in 1995 in 79 refineries indicated that the mean emission was 1,350 mg/Nm 3 OS 2. In 1998, 1,125 mg/Nm 3 was emitted. The reduction is attributed to the rigor of environmental legislation, as well as to the difference in emissions between Nordic and Mediterranean countries (550 mg/Nm 3 in Nordic countries and 1,870 mg/Nm 3 in Mediterranean countries, in 1998). You can see in the graph the origin by OS 2 discharge processes.
Finally, although oil refining generates pollution, it must be taken into account that the chain does not end in refineries, since, in short, the use of many of the products that are generated in it also produces pollution. Therefore, in some countries, the most polluting components of oil have been extracted in the refining process itself, since its location facilitates its control.
Therefore, to finish, we can say that we take a lot of advantage of oil, but…

Oil refining diagram. (Source: Encyclopedic Dictionary of Energy; EVE).
Key processes in refineries
Separation
Physical refining processes are used to separate the crude fractions. The molecules of the components are not altered, only separated by size or chemical family. The separation is done by different processes:
- Atmospheric distillation of crude. The liquid evaporates totally or partially, separating the condensed fractions at different temperatures: gases, naphtha, kerosene, medium distillates (diesel) from the gross waste (main fuel base). It is the most used technique.
- Desulfurization processes. For each type of distillate a separate desulfurization process is used (by hydrogen, by caustic soda stream…). The goal is to extract sulfur.
- LPG separation. Fractionation of separate LPG in distillation distinguishes between butane, propane and combustible gas. The latter is burned in the refinery kilns.
Conversion

Chemical refining processes are used to separate specific products from fractions. In chemical processes, the molecular structure changes: it decreases the size of the molecules (cracking or breaking), enlarges them (usually by catalysts) or becomes a molecule of another petrochemical family (catalytic transformation, reforming and isomerization, for example) without altering the length of the chain. The most common conversion processes are:
- Vacuum distillation of heavy fraction. Requires a temperature lower than that of atmospheric distillation. Heavy diesel and waste (sometimes asphalt) are obtained and each fraction will have a different treatment. It applies to the sand of atmospheric oil distillation.
- Thermal and catalytic cracking. Heating or treating waste with a catalyst breaks down hydrocarbon molecules. It is used to reduce residue viscosity, obtain oil coke, light and medium products and hydrocarbon vapor to be sent to fracture. If catalytic cracking is done with hydrogen it is called hydrocracking. Hydrogen is used to remove pollutants such as sulfur, nitrogen or metals.
- Catalytic reforming of naphtha. After desulphurisation, dehydrogenation and isomerization reactions increase the content of naphtha aromatic compounds and therefore the octane numbers of gasoline. The hydrogen abundance resulting from these reactions is used for the aforementioned desulfurization processes.
- Hydrogen production. In the classical refinery the hydrogen needed for desulfurization in the catalytic reforming unit was produced. However, when deeper desulfurization or higher quantities of distillates are required, hydrogen production must also be higher, which is achieved in steam reforming units. In them the hydrocarbon in H 2 and CO 2 is completely decomposed.
- Butane fraction optimization. From various butane currents present in the refinery, valuable liquid products of high octane number are obtained for the preparation of unleaded gasoline.
Sometimes physical and chemical processes are performed simultaneously, such as distillations with catalysts. The goal of mixed processes is usually to reduce the number of steps to take in the production unit.