Food additives

Since entering the European Economic Union, our food legislation has been tightening, highlighting the composition and labelling of preservatives. Royal Decree 2058 of 12 August 1982 regulates labels, etc., requiring that item 8.10 indicate food additives; for those used in the EC with the symbol E... or for those who do not yet have a number admitted by the symbol H...
The definition of additives can be done in many ways and we will use one of the most suitable for each case. The substances added to food by the additive are non-nutritious. The added quantities are very small, almost infinitesimal and their objective is none other than the remodeling or improvement of the elaboration and conservation of some of the organoleptic characteristics of food and / or drinks.
Additives such as salt, sugar, alcohol, maceration, dyes have always been used... Additives are chemicals whose origin can be natural or synthetic. We add additives to food to delay food breakdown, change flavor, etc. In general, natural additives can be considered more suitable than synthetic additives, although they differ only if they are toxic or not by organisms.
Additives should not be mixed with contaminants such as pesticide dioxane, pesticides added to tomato plants or those that caused the toxic syndrome of rapeseed oils. Of course, the effects of the residues of chemicals used as pesticides on our bodies, due to their cumulative nature, can be very noticeable. As a result, pesticides are not considered additives but contaminants.
From a chemical point of view, additives can be easily posed; the food itself undergoes morphostructural changes over time, reflecting some chemical changes and chemical reactions. Therefore, to understand the behavior of additives in food conservation and to defend their scientific views on it, we will analyze the processes that occur spontaneously in food.
Food degradation. Agents
There are three types of agents: physical agents (freezing, mechanical pressure, light, heat...), chemical (non-enzymatic, among which one can mention the anointing, the process of numbness and the finishing process; and enzymatic) and living (insects, rodents, microbes,...)

Different techniques or processes of food conservation can be used, such as heating, cooling, drying, etc., or chemical compounds (natural or synthetic) called additives to which they are appropriately added, such as the famous E-... The latter present some specificity for some chemical reactions that occur spontaneously.
Foods are based on chemical compounds, such as glucids, lipids, and protides. Glucids, lipids and protésids are components of food, consisting of different chemical compounds, such as carbohydrates, fatty acids and their glyceride esters (fats and oils), and proteins are formed by amino acids, respectively. In foods there are more compounds, such as vitamins, trace elements, etc. But since the ones mentioned above are the most numerous, we will analyze them below. All this is explained in the double issue 85-86 of this magazine.
Physical processes or techniques used in conservation (freezing, heating, etc.) are well known although we do not know exactly the complexity and technical explanation (for example, they are not necessary), we abandon them and we will limit ourselves to chemical changes.
The chemical processes that occur in food, mainly chemical reactions, are kinetic processes, so at higher temperature, higher reaction speed. As a result, in general, in summer the food rots faster than in winter.
Chemical changes in food
Drag process
This is the result of a set of complex reactions that occur in certain foods, exposing black or brown pigments. Volatile compounds that alter smell and taste are also produced. Often such effects and reactions are used intentionally, for example in the case of beer or cheese.

Not to mention the concrete process, it must be said that heat (temperature) has a great importance and influence. Therefore, in foods obtained by techniques at high temperatures (pasteurization, boiling, etc. ), like condensed milks or fruit juice, there is an enormous risk of burning.
From a chemical point of view, the aging process is mainly related to the carbonyl group (C=O) of sugars and vitamins. The enzymatic process of drag is generally performed in phenolic compounds and is directly influenced by light and heat. An example is the one that appears in the short term after cutting the potato, apple or banana.
These phenolic groups oxidize with oxygen, light and metals (also by their enzymes) and as a result of the reaction polymers called melanins appear. In foods of animal origin the finishing process is not carried out, but is common in fruits and vegetables and accelerates when a handling process or a blow occurs.
Acidic fruits, such as citrus fruits, do not contain oxidizing enzymes. In lemon, orange, pineapple, etc. the attention process is not carried out. On other occasions, chemical substrates and enzymes may be placed in different microcells, and if a blow occurs upon acquisition and the membrane breaks, the dragging process will occur (hit an apple and see what happens! ).
However, coloured polymers called melanins create a protective membrane that prevents the entry of posterior microorganisms. This phenomenon can be observed when cutting the banana.
Mining Process
The food irritation process is then chemically analyzed. In fact, the membrane process is only a chemical transformation of lipid compounds, which can be a hydrolytic process or a normal oxidation. It will therefore be produced in fatty foods such as oils and fats. The reaction product is characterized by well-known odor and taste chemical compounds.

In the so-called hydrolytic or lipolytic process the ester bond of lipids is broken. The speed of this reaction depends mainly on heat and enzymatic catalysis.
Remember that fatty acids are long aliphatic chain carboxylic acids and that the double C=C bond separates oils and fats from the chain.
In saponification reactions, well known for chemicals, molecules of esters and water are generated. Kinetically analyzed under standard laboratory conditions is very slow, but in nature thanks to enzymes is very fast.
The reverse process of this equilibrium is completely known, the hydrolysis of esters, and is a very slow process even studied kinetically. However, those catalyzed by enzymes (proteins) are much lighter. Do not forget that in these kinetic processes the temperature influences a lot. We estimate that by passing the temperature from 25ºC to 100ºC, the reaction speed increases approximately 50 times. Therefore, as it is easy to understand, it is a factor to consider in the production of dairy products.
This hydrolysis is common in butters. The spicy taste and smelling smell is due to butyric acid.

Reaction products are fatty acids that, logically, will acidify the pH to the extent that they are acidic and that, along with their smell, will alter their organoleptic characteristics. Therefore, it is no coincidence that to minimize the content of fatty acids, the refining of the oil used for food is done.
Although what has been said so far has been due to the hydrolytic membrane process, there is an oxidizing membrane process that is carried out thanks to oxygen in the air that oxidizes unsaturated fatty acids. Moreover, if instead of combining these unsaturated fatty acids (i.e., trilezric compounds) are free, their self-oxidation is faster. In fact, this process is a radical polymerization (which we will not chemically analyze).
Heat, light, metals and ionizing radiation like thunder are catalysts. As a reaction product we have everything: short aliphatic chain fatty acids, aldehydes, esters, peroxides, ketones... Some of these substances can be toxic so no hot oils/fats are used.
Antioxidants
To prevent the oxidation of lipids are added to foods antioxidant substances. Your goal may be to remove free radicals or remove peroxides. However, they must meet a number of requirements: not to be toxic, not to give tastes, smells and colors, act in very small amounts and, of course, be fat-soluble.
Natural antioxidants include tocopherols (vitamin E). Among the synthetic antioxidants, the most commonly used are BHA (butilhydroxyanisol) and BHT (butilhydroxitoluene). Wood smoke, containing phenolic compounds, appears to have protective behaviors.
Before moving forward, we will explain the basic concepts of microbes and microbiological changes, since it is difficult to understand the microbial population growth and its dependence on concentration, temperature, pH, humidity and oxygen, such as cooling (its effect on the cellular structure), freezing (light or slow) and drying (solar, industrial, lyophilized).
Microbiological changes in food
We will present this topic in a somber and agile way to facilitate the understanding of both experimental techniques and the behaviors of some additives used in conservation.
Microorganisms, like us, are heterotrophs, so we face each other. That is, the food of these microorganisms is ours. Like us, microorganisms must also eat to satisfy all energy, plastic and chemical functions. In addition, we cannot forget that some of the microorganisms can be pathogens, such as salmonella. Microorganisms or microorganisms can be: (with separate nucleus, such as protozoa, fungi, algae...) and prokaryotic microorganisms (with undifferentiated nucleus, such as bacteria, cyanopas...).

Those responsible for food changes are mainly bacteria and fungi. The population growth of these microorganisms is exponential, that is, of a cell two new ones are generated, each of them will generate two others, etc. The population of bacteria in less hours can be one million. The techniques or procedures used in conservation consist in the interruption or inertization of the growth process of these microorganisms.
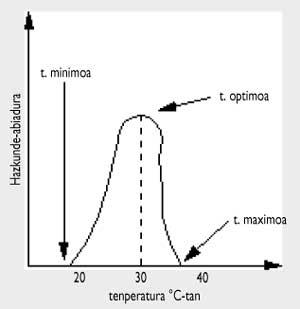
As can be seen in the attached figure, temperature greatly influences the growth of microorganisms.
The maximum growth speed occurs at optimum temperature. If for a period of time we heat the food above the maximum temperature, the microorganisms will die. This is the heating technique for conservation. Below the minimum temperature most bacteria “freeze”, that is, do not die, but do not reproduce.
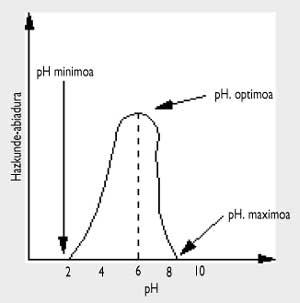
In the attached figure it can be observed that acidity or pH is also important. In
general, both molds and yeasts can withstand a more acidic pH, while when pH<4.5 bacteria will not attack. Some foods are very acidic, like fruits, and others can be preserved by adding vinegar.
The basic component of microorganisms is water, which without it dies, so there is no growth. In the case of temperature and pH, for each microorganism there is a maximum activity (aw), minimum and optimal of the water, where the maximum activity of the water, the vapor pressure of the water in dissolution is divided by the steam pressure of the pure water.
Most bacteria cannot grow in a medium with a maximum water activity of less than 0.90, so based on this property in food conservation techniques are used such as water reduction (by sublimation or evaporation), freezing and reducing the maximum speed by dissolving salt or sugar.
Additives or additives
As mentioned at the beginning, the aim of this article has not been to explain experimental conservation techniques, but to explain the additives or additives used for food conservation and to comment on their causes.
From what has been said so far it is easily deduced that food is “rotting” or decomposing, that is, that chemical reactions occur within it and that the growth of microorganisms can be evident. Therefore, procedures or techniques can be used that allow maximum conservation of food, including chemical additives or additives.
Additives are chemicals that are intentionally added to food and that modify, modify or stabilize the organoleptic characteristics of food. Strictly speaking, sugar, salt, vinegar, etc. that are used in preserves, although they can be considered additives, we consider them as chemical preservatives. That is, substances that do not alter the organoleptic characteristics of food, but also delay or eliminate the microbial presence, will be called preservatives.
As stated above, any additive must be authorized. The list of additives used in Spain includes dyes; preservatives; antioxidants and synergists; emulsifiers, stabilizers, thickeners and gelling agents; acidifiers and acidity correctors; antimalutants; flavor enhancers; foams; artificial sweeteners; modified starches; gasifiers and others.
Instead of describing each additive, this long list will be grouped together and the most important chemical characteristics of the set will be indicated.
Colorants
They are used to color and/or decorate food. They are additives from the E-100 to the E-175. In fact, dyes have no technological or physiological interest, and their use focuses on consumer demand. Some technicians consider dyes as the best example of superfluous additives, as they only change their appearance.
In general, it is said that natural dyes and their derivatives (such as carotenoids, xanthophylls, chlorophylls) are not toxic, while synthetics, especially azoic dyes (E-102, E-105, E-110, ..) can produce side effects. The chemical structure of a azoic dye is as follows:
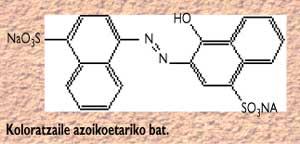
These are
the railway lines that go to Calle San Martín. The most commonly used are benconic acid (E-210), sorbic acid (E-200), acetic acid (E-260) and proton (E-280) and their salts and nitrites, nitrates and sulfites.
Benzoic acid and its derivatives (E-214, 215...) (known as parabens) are probably the most used, and their structure is as follows:
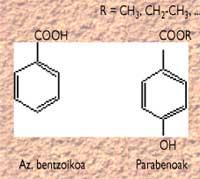
The antimicrobial effect is due to the non-dissociated species of all these acids, so they are effective in pH acids (between 2.5 and 4.0). They are not toxic in the usual concentrations used in conservation. However, they are not very suitable for our body in high concentrations, so it can happen that salicylic acid that was previously used (which can be considered as a benconic derivative) was toxic and that today is not used practically if it is not kept at home.
Acetic acid CH3-COOH, i.e. vinegar, is widely used. Sodium and potassium salts of nitrites and nitrates (from E-249 to E-252) are used in meat products, mainly sausages. The work of these salts is triple: on the one hand, they develop a special color in the formation of nitrosylmyoglobin, on the other, they prevent the growth of Clostridium botulinum and, finally, give the flavor a special “hint”.
Antioxidants and synergists
Its objective is to prevent oxidation processes that occur in fatty or oily products.
The most commonly used are the BHA (E-320) and butilhydroxitoluene BHT (E-321), whose structure is as follows:
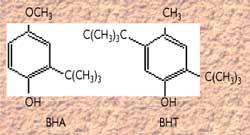
In this ring the presence of the hydroxylic group is important as it is a hydrogen donor. They are not toxic or give special flavor.
Some antioxidants, such as sulfite and sulfur oxide (from E-220 to E-226), also have conservative properties and are widely used in winemaking.
Emulsifiers, stabilizers, thickeners and gelling agents
Many foods, such as milk or egg (yolk), are naturally dispersed systems, that is, they are placed in two immiscible phases such as oil and water. As could not be otherwise, there are special chemicals that decrease the surface tension of the water and many that homogenize both phases. These substances are called surfactants, emulsifiers, surfactants, etc.
The chemical structure of these surfactants or emulsifiers is very similar in most cases; the hydrocarbon aliphatic chain, soluble in the organic phase and often called hydrophobic or lipophilic, and the soluble ionic end in the aqueous phase, also known as hydrophilic.

The industrial use of emulsifiers is often applied to water/oil emulsion, such as ice cream, mayonnaise, margarines. Milk itself and other foods contain this type of emulsifiers, such as lecithin, cholesterol.
In bakery is known the property of starch to crystallize. Therefore, in this type of “gels” (mixtures) are used molecules called gelling, such as stearil-2- lactilate sodium (E-481). This additive reduces the rate of hardening of bread.
The action of phosphates (E-450) is multiple, since they stabilize emulsions, are shock absorbers at pH and can be moisturizing by capturing water. The most commonly used are hydrogenophosphates (Na2HPO4), dihydrogenophosphates (NaH2PO4), polyphosphates, sodium hexametafosfate (Na6P6O24), sodium pyrophosphate (Na4P2O7). They are widely used in butcher products.
Acidifiers and acidity correctors
Acids used in foods, such as fumaric (E-296; E-297), acetic (E-260), adypic (E-355), phosphoric (E-338), citric, etc., can also be considered conservative. The source of most acids is natural (apple, banana, carrot...) and in addition to being preservatives, they are pH absorbers and synergistic to antioxidants. Probably citric acid is the most common and used.
All acids give a special and clear flavor to the food. These acidifiers are not toxic.
Other
Food processing, in addition to those already mentioned, often uses many other additives. Its function and function, so to speak, has been to “decorate the product” or choose it. Among them are flavorings (E-620 to E-637) that allow to recover the taste that food has lost through industrial processes. Aromatizers can be synthetic or natural.
In addition, the industry uses additives such as antienlazantes or antimalutantes (from E-535 to E-573), starches (from H-4381 to H-4394) or sweeteners.