Clean or kill air

The gas mask is the one that receives clean air from a bottle that is carried on the back. J. Lane's invention carried compressed air in the bottle.
The diving system is the same as that of firefighters. Among the smoke from fires there is usually very little oxygen, so even with the cleaning of the outside air there would not be enough oxygen to breathe properly. That's why firefighters use autonomous gas masks.
At the beginning of history, the goal of bottled systems was similar to that of outdoor air washing systems; to be able to walk or work between fumes or ‘dirty air’.
Today, however, normally, the name gas mask is used to designate masks that clean contaminated air. They are placed on the face, some cover only the mouth and nose and others cover the entire face to increase protection.
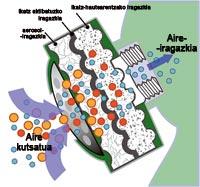
Gas masks remove pollutants from the air and make the air breathable. Toxins in the air can be gases or particles and are protected by gas masks.
The three ways to remove hazardous components are filtering, absorption or adsorption and reaction. But in any case, the oxygen that is finally breathed is the one in the contaminated air.
Filtering
This system is the simplest of the three and extracts particles from the air such as bacteria, viruses or spores.

Surely you have ever used a scarf to protect you from dust. This is, in short, a filter against particles in the air.
In rag or mask, the filter holes should be smaller than the particles in the air to prevent their passage. Gas masks filter particles greater than 0.3 microns.
Logically, blocking the filter requires modifying it, but it has another limit. The smaller the holes, the more strength it takes to pass the air and breathe. Therefore, gaseous contaminants, much less than particles, must be extracted by other systems.
Absorption or adsorption
Absorption is the absorption of one substance by another. Adsorption, however, is that a solid maintains gaseous or liquid molecules or ions on its surface.
filters are clogged, the filter should only be changed.
The influence of one or the other is the same: the polluting substance is attached to the filter.
Active carbon is very appropriate for the capture of organic matter. It is a carbon treated with oxygen, with millions of small pores between carbon atoms, with a surface of between 300 and 2,000 m 2 per gram, widely used to capture substances that give odor or color to gases or liquids.
Zeolite is also widely used for the extraction of organic and inorganic compounds. Natural zeolites are hydrophilic minerals formed by aluminum, silicon and oxygen.
While a chemical reaction can occur in this filtration system, it is not necessary, it can work because adverse electrical charges are attracted.
The same effect is achieved when a handkerchief is wet and placed in the mouth and nose to protect itself from smoke. The smoke will be absorbed by the water in the handkerchief and a cleaner air will reach the airway.
Reaction
This third form of mask cleaning is based on harmful substances reacting more easily than air.
Reagents, such as acidic substances, are used to react with toxins. The reagent is attached to a bracket or matrix as if it were a lid. Many times the support is resin and, the more porous it is, the
greater its surface, the greater the amount of acid that can be glued as coverage, which will allow filtering more air.
Upon entering the mask, the toxin reacts with the reagent and is neutralized.
Source of fetishism
Not many, but there are those who use gas masks for sexual fetishism, especially in the UK. You have to find an explanation for all this and it has been said that it can be a child imprinting, masks II. Since its use in the World War. But this does not explain the case of those who were not children in that war. An option may be to be part of the so-called ‘rubber fetishism’ or that the non-human appearance wearing gas mask generates erotic fantasies.





