Conductive polymers
Some polymers are able to drive electricity. One of them, poly (sulfur nitride), can replace gold in electronic devices. But this material has problems in its processing. Chemists discover a new way to make poly (sulfur nitride).
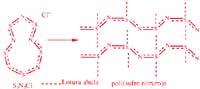
Arthur Banister and his colleagues at the British University of Durham have achieved polymer through a dissolution of the compound S5N5+Cl. The tetrasufre tetranitride of poly (sulfur nitride) was previously used as raw material. This compound is explosive and dangerous.
In order for the polymer to generate electricity, it must have a special structure: in its skeleton double or triple links must appear. Electricity is driven through these connections.
In order for the polymer to be a true conductor, electrons must circulate as easily as in metals in their structure. Movement will occur in polymer chains and between chains. In poly(sulfur nitride) electrons circulate by double nitrogen/sulfur bonds and between chains by means of sulfur bridges.
It was first synthesized in 1910 and its metallic appearance was checked. Until 1975 it was considered a chemical curiosity, but that year IBM chemists saw that it was superconducting at 0.27 K. Poly (sulfur nitride) is a real metal, although it does not contain metal atoms in the structure.