Nobel Prize in Chemistry 2014 for those who converted microscopy into nanoscopy

0.2 micrometers. In 1873, Ernst Abbe placed the limit of the smallest structure that could be seen with the microscope. It was a physical limit conditioned by the wavelength of light. This allowed to see cells, but not molecules much smaller than these, such as proteins. According to the Royal Swedish Academy, the work of the three award-winning researchers has given a “smart solution” to the border and, since then, there is no too small structure.
Fluorescent molecules are the tool of this ingenious solution and, two, the principles that support them. The first, called STED microscopy, was developed by Stefan Hell. The other is the contribution of Eric Betzigen and William Moerner. The three awarded for developing a high definition fluorescence microcopy.
Nanolinterna by Hell
At the University of Turbo (Finland), Stefan Hell had the idea of Nobel Prize when working on fluorescence microscope. Fluorescence microscope uses fluorescent molecules that are associated with specific cell molecules; fluorescent molecules, when excited with light, emit light, so researchers are able to locate the molecules they want to see. But then the resolution of the technique was too low, for example, to separate the strands of DNA. To achieve greater definition, Hell proposed that you could use the excited broadcast.
Thanks to the excited emission, scientists are able to turn off fluorescent molecules to their liking, using a laser beam. One STED microscope works like: one laser excites all fluorescent molecules in the sample and the other turns them off, except those found within a nanometric area in the center of the sample. The signal is recorded and the process is repeated in another area of the sample. This way the entire sample is covered with nanometers, obtaining a high resolution image.
Helle presented the idea in 1994 and gave him a six-year job of developing the STED microscope, the first result was an image of an E. coli bacteria. Tripled the resolution of the conventional optical microscope.
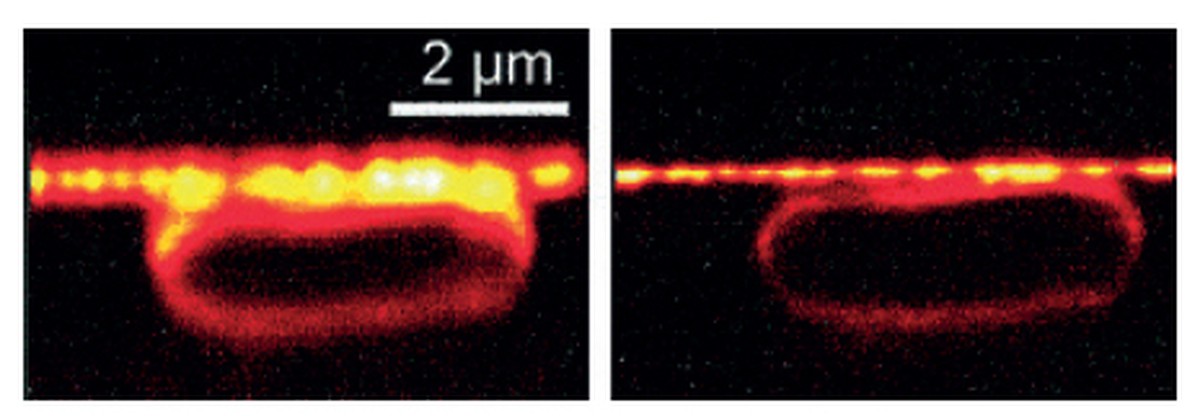
Moerner and Betzig, trying to turn the molecules on and off separately
Like Hell, Betzig and Moerner went to fluorescent molecules to overcome the limit of Abad. Both have developed separately the bases of monomolecular microscopy, built by Betzig on Moerner's work.
The method is based on fluorescent proteins that can be switched on and off on demand. This method consists of illuminating several times the same field and exciting a small number of fluorescent molecules, so that, after repeating the process several times, all the received images are grouped into a single image obtaining a high resolution image.
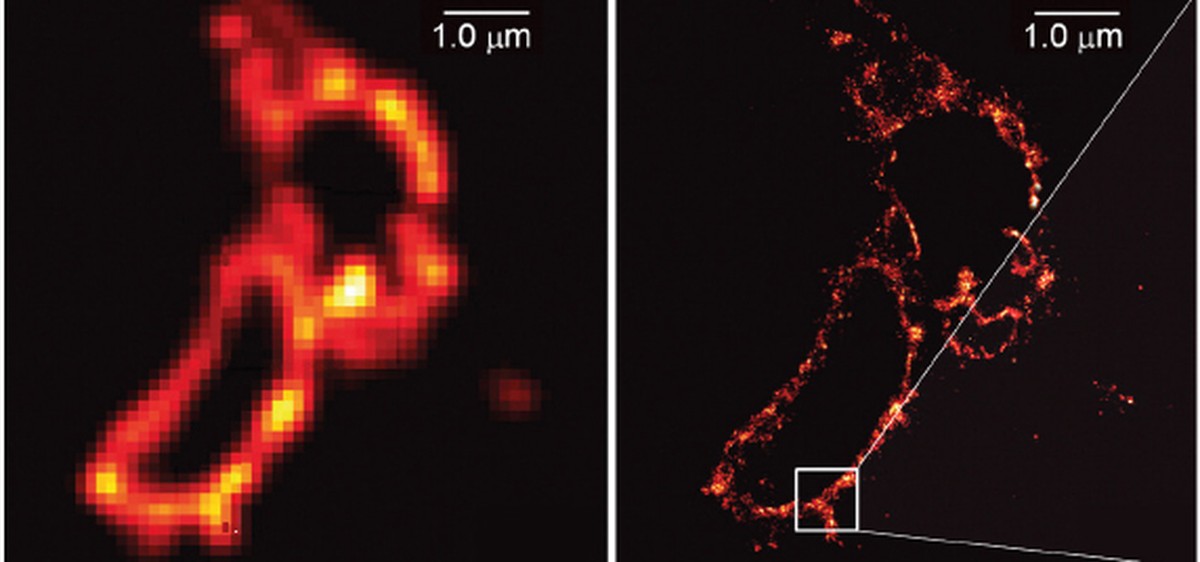
The first image obtained by this method was presented in 2006 by Betzig, a lysosome. But the work of both has been indispensable. In fact, Moerner was the first researcher to measure single-molecule fluorescence in 1989. He also discovered the GFP fluorescent protein variant that can be switched on and off freely through the light. In short, Moerner discovered that the fluorescence of individual molecules can be optically controlled and Betzig converted it into image.





