Synthetic life?
The first artificial molecule that can be repeated has just been synthesized in the US. The new molecule is much simpler than repeated biological molecules, such as DNA. The chemists who synthesized the new molecule have said “At least it is a primitive sign of life.”
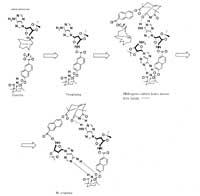
Julius Reb and his colleagues at the Massachusetts Institute of Technology (MIT) have synthesized the molecule. The molecule called AATE acts as a temperate and combines pieces of molecules to make the copy of the original molecule. It is similar to the DNA process. However, in the latter case it is necessary to work an enzyme to start the process.
Reb and his co-workers have combined the aminoadenosine with an ester in chloroform at room temperature and subsequently added triethylamine. In reaction the molecules come together synthesizing AATE.
AATE, to be repeated, attracts the ester molecule to the adenosine end and the aminoadenosine molecule to the ester end. Both sides then react to each other forming a new aate molecule.
Hydrogen bonds allow the process. Each end of the AATE knows its “friend” through a couple of hydrogen bridges. Both sides can react when fixed to the tempering to form a new aate molecule. Then the two parts are separated.
The speed of reaction depends on the speed of separation of the two aate molecules. This speed is low due to the breakage of four hydrogen bridges. Enzymes work in nature, but in the case of AATE, thermal vibrations release molecules. Rebbe studies how to accelerate the process.
To say that the molecule repeats itself, three arguments have been used. First, the AATE catalogues the synthesis of itself. Secondly, two aate molecules are inserted together as two pieces of a puzzle. And thirdly, when one of the hydrogens that build the bridge has been blocked (using a methyl group), the reaction speed has slowed considerably, as AAT has trouble knowing the ester or adenosine molecules.