Archaeological dating
The age of archaeological remains is known through different systems. In the last ten years, the base of 14 radioactive elements of carbon and dendrochronology (based on the study of tree trunk rings) have been widely used as a complement.
Radioactive carbon
Thanks to the Carbon 14 method, in the mid-1960s, the dating that until then was accepted for European prehistoric remains was upside down. At the Olduvai site in Tanzania, it was determined that a layer was 1,750,000 years old. The tools found are the oldest so far and it can be said that humanity has at least those years.
However, the lack of accuracy of carbon dating 14 has now been observed. The radioactive isotope of carbon 14 is only the trillium of all the carbon existing in the world (1/10 12). A very low proportion. However, this means that for every gram of normal carbon there are 60 billion carbon atoms 14.

For every gram of normal carbon, 13.5 carbon atoms 14 are disintegrated every minute, i.e. one atom every four to five seconds. This happens with the current carbon (for example, the one we have in the skeleton of our body), the organism that constantly represents the disintegrated radioactive carbon atoms. In dead fossil organisms, radioactive carbon decreases as it disintegrates. Every 83 years it has 1% less radioactive carbon and at 5,730 only 50%. (This means a half-disintegration period of 5,730 years.) Then there are 6.78 disintegrations per minute and gram. At the age of 22,920, an atom per minute disintegrates and at the age of 40,000 it is necessary to wait about ten minutes to detect a disintegration. From there, radioactivity is very weak and therefore difficult to detect.
Lack of precision of radioactive carbon
However, the semidisintegration period of this isotope has not always been well fixed. It was considered that in 1940 it was 25,000 years, in 1950 between 4,700 and 7,200, and later Willard Libby, discoverer of the system, claimed that it was 5,568 years. In 1962 it is also said to be 5,730 years old. However, the Libby figure has been used to date and has been accepted by scientists, although to know exactly the age has had to multiply by factor 1.03.
In addition, the age of current samples is calculated and it is known that in the last forty years atomic explosions have caused a significant increase in the rate of radioactivity in organisms (around 3%). The industrial revolution has allowed the emission of non-radioactive carbon into the atmosphere, which has reduced the rate.


The exact determination of age is not so simple. Radioactivity is a random phenomenon. Beta particles received by the registrar do not arrive regularly. In case of dispersion, the standard deviation is calculated. There is a 68% probability that the estimated age is between +1 and -1 of the actual age. If tolerance is between +2 and -2, the probability is 95.5%. Therefore, the figures given today are usually accompanied by tolerance. The graphics also mark the tolerance strip next to the age point.
At the age given by laboratories we must add 3% per period, then the years since 1950 and third tolerance. But it's something else. In the beginning it has been considered that the formation of radioactive carbon in the upper part of the atmosphere was constant, but in reality it has not occurred, since it depends on the activity of the sun. The Sun has a cycle of eleven years and in the formation of radioactive carbon there have been variations between 1% and 3% in recent decades.
All these inaccuracies, like the Egyptologists, were not at ease with the dates given to them. In some cases the deviations from their calculations were 700 and 800 years, but the radioactive carbon dates were always “younger” than historical chronologies.
Dendrochronology supporting carbon
The lack of precision of radioactive carbon has been partly resolved by dendrochronology (dating system by analyzing rings on tree trunks). This method takes the name of the Greek word dendro (tree). In the southwest of the United States, some desert pines (type Pinus aristrata) have been able to last for thousands of years and, analyzing their trunk rings, has been able to build a chronology until prehistory (approximately seven thousand years a.C.).What has basically been done is to take wood samples of a known age and date with a radioactive carbon 14 method. In other words, the radioactive carbon method has been “calibrated” with dendrochronology until 6000 BC. It has been proven that carbon calculations were accurate. in the first centuries but a. C. at the time gave younger dates: a.C. 200 younger years by the year 1000, 400 younger years by the year 2000 and 800 younger years by the year 5000.
Shroud of Turin

The calibration and elimination of “background noise” currently allow to calculate the dating of medieval samples in 20 years. In addition, with the use of particle accelerators, the milligram sample is sufficient and some parts can be manufactured without breakage or deterioration. Dating the Shroud of Turin has been a success, but at first it had some drawbacks. Three laboratories dated the radioactive carbon method, but given the incidents in the calibration curve, the actual date was XIII. could be the second half of the century or more than a hundred years later. The first historically made mention of the Shroud dates back to 1350, making it the 13th. For a sample analyzed in Lyon a dating was given between 1264 and 1283.
Uranium-thorium system
The disintegration of the Uranium 234 isotope becomes a 230 thorium, a transformation that can be detected by a new method of mass spectrography.
A group of American scientists have taken samples up to 124 meters deep in the corals of the island of Barbados. When the last glaciation was booming, the ocean level was 120 meters below the current one. Since then the corals have been rising with sea level and, dating the corals with the uranium-thorium system, it has been possible to build its history, closely related to the history of the climate.
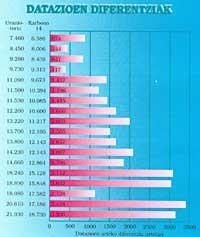
The climatic oscillations achieved for the last eight or ten thousand years have been compared with those indicated by dendrochronology and it has been proven that they are practically identity. Continuing with uranium-thorium coral dating until 20000, the carbon 14 method has been “calibrated” until dendrochronology does not cover. From the year 6000, it has been observed that in the glacial epoch the carbon system has given differences between 2,000 and 3,000 years. All prehistory must therefore be “aged”. In Lascaux, for example, the radioactive carbon method calculated the local wood an age of 17,000 years, but it actually has 20,000.
Other systems
In archaeology different dating systems are used depending on the age and nature of the sample, and sometimes several methods of analysis of the same sample. Archeomagnetism especially ceramics (containers, ovens, etc.) It is used to date and has an approximate validity of 6,000 years. As for dendrochronology, it can reach 7,000 years (although only for the study of wood) and radioactive carbon up to 40,000 (for the dating of living organisms). From there the system of potassium argon and thermoluminescence is used, one for the dating of volcanic layers and another for materials that have been heated a lot (containers, oven stones, volcanic stones, etc.) to calculate.





