What radioactive elements were poured out and how do they reach the sea?
It has been just over five years since the triple accident in Japan: earthquake, tsunamis and nuclear accident. On 11 March 2011, the Tohoku earthquake and subsequent tsunami violently affected the Fukushima Dai-ichi nuclear power plant, causing the largest abrupt discharge of radioactivity to the sea to date. In fact, the spill of Fukushima was five times lower than that of Chernobyl and 50 times lower than that of dry testing with nuclear explosives (1945-1980). However, concern persists because radioactivity has not been reduced as quickly as imagined in coastal sediments, biota and water, a sign that the Fukushima plant has emitted continuous spills.
Marine researchers from the Autonomous University of Barcelona and the Institute of Environmental Sciences and Technologies, together with scientists from Japan, Europe and the United States, are analyzing the influence and evolution of the Fukushima accident at sea. Since June 2011 we have gone every year to the coast of Fukushima to take samples in an oceanographic boat: water, sediment and biota (zooplankton, rays, algae, etc. ). ). In the laboratory, in 2011 radionuclides (radioactive elements) were measured, which caused the radiological risk and were emitted in the largest quantities. That is, those who have a short half-life (strontium 89, sio-134, iodo-131, etc. ). ). In the following years, when the latter were unused due to radioactive decay, we have investigated fission products that persist for decades: sio-137 and extronura-90 in particular. In fact, Fukushima radionuclides offer an excellent tool for understanding terrestrial and marine geochemical processes and the relationships between the two media. For example, literary data and ours have suggested that isotope assignments (cesio-134 and cesio-137) issued by Fukushima had arrived (and arrived) at sea in different forms and deadlines.
In March 2011 most of the emissions were gaseous. The earthquake and tsunamis destroyed nuclear fuel cooling systems. This caused an increase in temperature of 3 out of 6 reactors, accumulation of radioactive gases and explosion. Most volatile radionuclides (cripton-85, iodo-131 and 129, xenon-133 and 135, cesio-134 and 137, etc.) in coastal waters and in open sea.
Contaminated water, also used as a cooling emergency for reactors, was poured into the sea by TEPCO (Tokyo Electric Power Company), the nuclear power plant management company, in April 2011. In the case of the cession, the liquid discharges were 5 times smaller than the aromas. In non-volatile radionuclides, for example, contaminated cooling water has been the main source. After these first liquid spills in 2011, the Fukushima plant has suffered more leaks, although TEPCO has surrounded the plant and built several walls to protect the coast.
The last two springs are rivers and groundwater. About 20% of airborne radionuclides were deposited in Japanese soil. Out of this total, about 2% of the cesio-137 has left the sea, retained in the sediments that carry the rivers. Groundwater carries a similar amount. The amount of extra-90 emitted into the air was a thousand times lower, so it barely reached the sea.
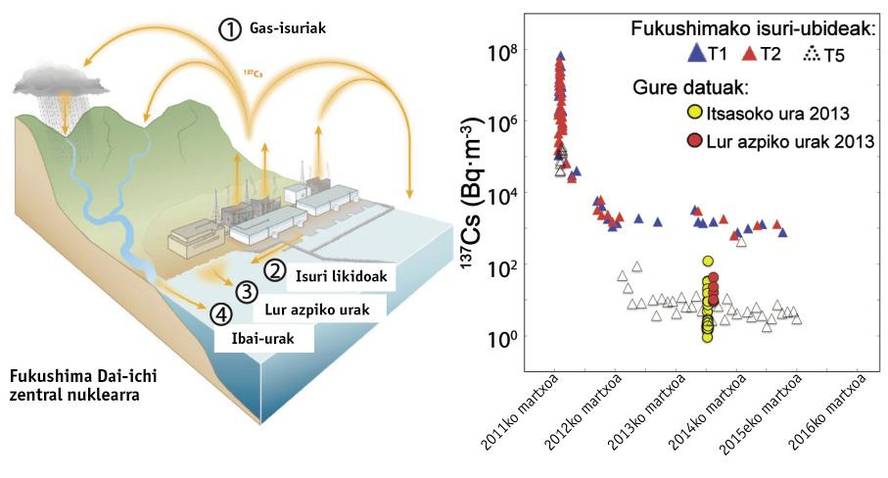
To understand the influence of these four springs (gas, reactors cooling water, rivers and groundwater) on the sea, we have measured from Fukushima, on a water surface between 800 and 110 kilometers, and from the surface of the sea to 500 meters deep, between cesio-137 and extra-90. Below are our results and TEPCO concentrations in the discharge channels of the nuclear power plant. Concentrations are given in Bq/m 3. That is, the amount of Bequerel (Bq = disintegration per second) per thousand liters or cubic meter.
Evolution of radionuclide concentrations
In the coastal waters of Japan the concentrations of cesium-137 and extra-90 were 1-2 Bq/m 3 before the accident, due to tests conducted with nuclear explosives. In April 2011, liquid vapors and spills increased the concentration of cesium-137 to 68 million Bq/m 3 and strontium 90 above 100,000 Bq/m 3 in the spill channels around the central. In the following months concentrations decreased markedly, the first to 10,000 Bq/m 3 and the second to about 1,000 Bq/m 3. Since then, concentrations have undergone significant variations. In contrast to concentrations prior to the nuclear accident, often the cesio-137 between 100 and 1,000 times higher and strontium between 10 and 100 times higher between 2012 and 2016. In the water measured by us, almost all samples had signs of accident. In September 2013, for example, strontium concentrations 90, sio-137 and sio-134 were 9, 124 and 54 times higher than pre-accident concentrations. Similar or higher concentrations in water samples from the same site have been measured in 2014 and 2015.
The persistence of these high concentrations in water years after the accident makes clear, although to a lesser extent, that the plant continues to emit radioactivity. For example, a daily emission of 2,3-8.5 GBq (billion Bequerel) of 90 strontia was estimated in September 2013. This continuous discharge would involve between 100 and 1,000 times the river transport. In addition, it should be noted that thousands of liters of water are currently used to keep affected reactors at low temperature. The contaminated water that has touched the fuel is pumped, stored in giant tanks and treated to extract the maximum of radionuclides. After treatment, however, there are radionuclides that remain in the water: 90 strontium in part and tritium in its entirety. In 2015 there were some 600,000 tons of water stored in tanks, increasing the risk.
In addition to water, key-137 is accumulating in coastal sediments (around 1% of 2011 emissions). According to the latest work, in coastal sediments and, above all, in the area of Fukushima, in 2016 there are between 5 and 10 times more transfers than in native waters. To reduce this amount in half it would be necessary to spend at least 10-25 years.
Effects on marine biota
Marine animals quickly penetrate cesium into muscle rather than potassium and strontium into bones. A few weeks after the accident, high concentrations of cesium were measured in sediments and fish living near the plant. However, these concentrations were normally below safety limits (500 Bq per kilo of fish in 2011). To reduce the distrust of Japanese society, they sharpened security and reduced the limit to 100 Bq/kg, well below the European border (1,250 Bq/kg). In 2015 only 1% of the concentrations exceeded 100 Bq/kg. The downward evolution has allowed the exploitation of several species of fish that were prevented in 2011 and revitalize one of the most powerful economic sectors of Japan, fishing.






