Synthetic photosynthesis
A team of American researchers has broken a new path of solar uptake. An artificial molecule has been synthesized that mimics photosynthesis, the process by which plants obtain energy from the sun. The photons of light electrically lack the molecule. This charge is also quite durable and can be stored in an electric circuit.
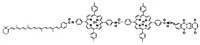
This molecule, called Pentado, has been synthesized in a laboratory at the University of Arizona. When a photon touches the molecule, it excites and an electron passes to the higher energy state. The electron is one of the porphyrin rings in the center of the molecule.
When the excited electron returns to its lower energy state, it moves to an adjoining atom. If you want to go down, jump to another atom and in this way, the jump move ends the electron away from the starting point. The jumps carry the electron to one end of the molecule and it is polarized. One end is positively charged and the other negatively.
Through the separation of loads, half of the energy of the photon is stored, slightly from top to bottom, in the form of potential chemical energy. Plants and some bacteria use the separation of loads to initiate the chemistry of photosynthesis.
Similar molecules have previously been synthesized, but in these cases, the separation of loads only lasted about 300 nanoseconds and then energy was lost in the form of light or heat. 2.- This time is relatively long to store the conductive energies of the molecule or to store them by other chemicals.
If the pentate is placed through a membrane (touching the two ends of it), the load can produce chemical reactions. These chemical reactions can store energy as occurs in batteries or batteries. If the molecule is placed between two conductors, electrical current is generated in an external circuit.
These molecules can be very useful. As Gusta says, “Illumine them and get the load; tie the ends with a thread and you have current”.