Phosphorus and chemical bond
A new chemical compound division is questioning the theory of interatomic bonds. German and British laboratories are synthesizing new phosphorus and carbon molecules. Connection theories cannot talk about the unique structures that are formed
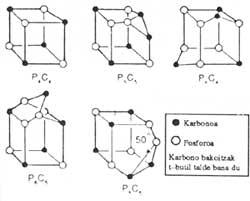
reduceManfred Regitz of the University of Kaiserlaurten (Germany) and his colleagues have just synthesized a molecule in the form of a phosphorus and carbon cube. Phosphorus and carbon atoms are arranged alternately at the vertices of the cube. The molecule has been called phospfacubano. T-butylphosphatethylene (phospfa-3,3-dimethylbutane) for phospfacubo synthesis has been heated for 65 hours to 130°C.
Meanwhile, Ulrich Zenneck and his colleagues at Heilderbe University have synthesized four other similar phosphorous hydrocarbons. One of them consists of a central set of six phosphorus atoms and five carbon atoms, with a cube structure in the form of a superior atmosphere.
This molecule and phosphor contain analogous molecules formed entirely by carbon atoms: homopentenoprisman-cube, respectively. Both molecules were synthesized in 1976 and 1964 and were considered a triumph of the capacity for organic synthesis.
Phosphorous hydrocarbons are more easily synthesized than carbon structures similar to yours and some do not contain carbonate analogues. Phospfocubano is not a perfect cube; the angles at the vertices of the carbon atoms are slightly depressed, with 94º.
Among the molecules manufactured by Zenneck, one contains 5 phosphorus atoms and 5 carbon atoms. Part of the structure is a triangle formed by three atoms and an angle between links is less than 50º. This type of organization puts electrons closer than enough and turns upside down all union theories.
In Britain, at the University of Sussex, John Nixon and his colleagues have synthesized similar amazing molecules. One of them, with six phosphorus atoms and four carbon atoms, is cube shaped with an extra atom on both sides. All these molecules have been synthesized in t-butylphosphatilene. This molecule is intrinsic. And because, as the theory questioned, the chemists thought that it could not exist.
The molecule has a triple bond between a carbon atom and phosphorus, and until recently it was considered that the triple bond can only occur between the elements of carbon, nitrogen and oxygen. According to the theory, the triple bond between carbon and phosphorus cannot exist because electronic orbitals cannot be combined with each other and, moreover, if a carbon atom is replaced by a phosphorus atom in a structure, it would be impossible for the same structure to persist.





