Individual vision and research of molecules in the DIPC
Researchers from the Donostia International Physics Center and the Materials Physics Center have managed to see, represent and analyze molecules individually. Two studies have obtained images of unique molecules using different techniques and from different starting points. Both research has been published recently in the journals Nature and Science.
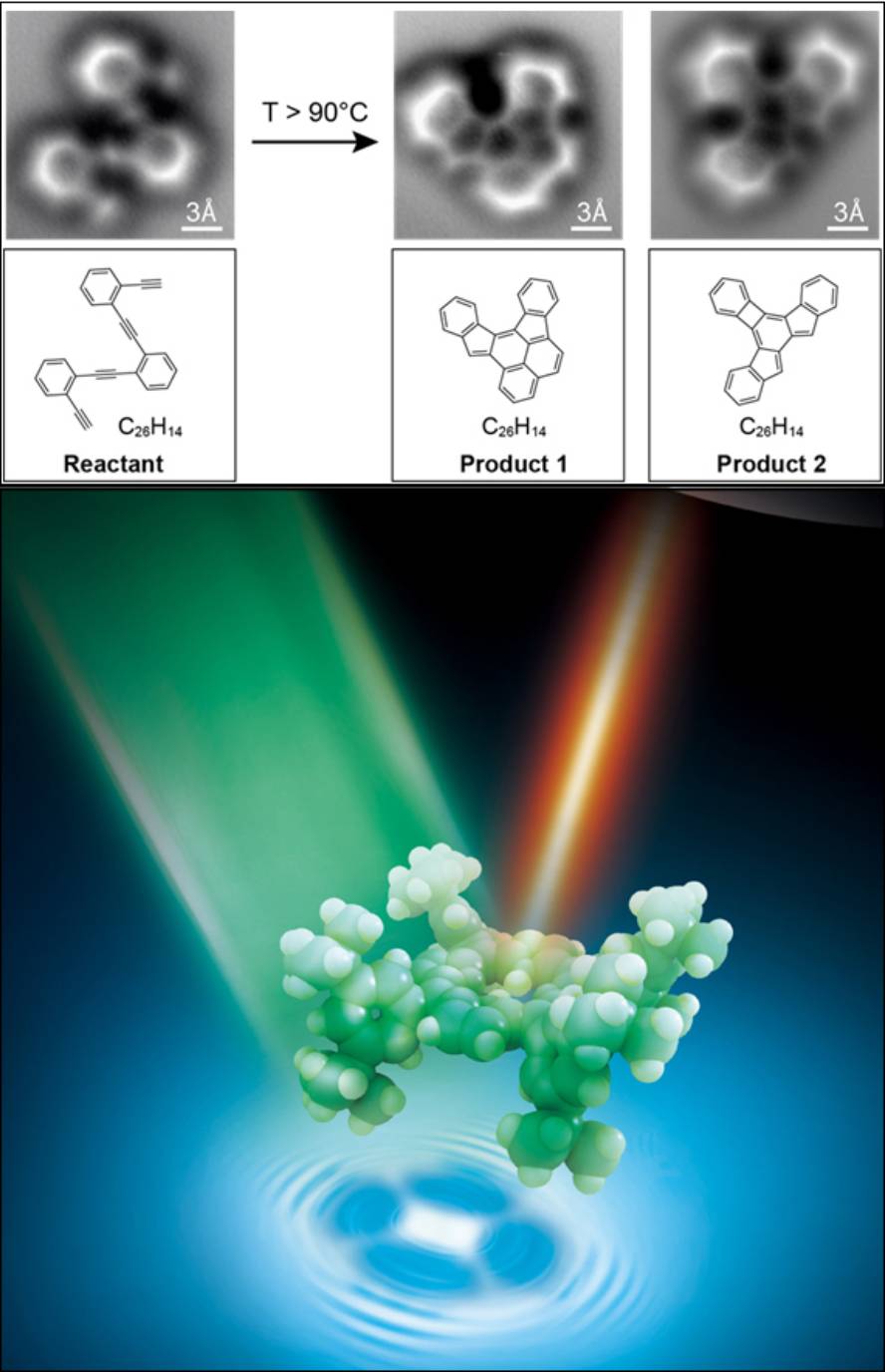
In the research published by the journal Science, we have been able to directly check the changes suffered by a molecule in a chemical reaction, that is, the processes of rupture and formation of links between the atoms that form it. Researchers at the University of California at Berkeley and San Sebastian have conducted this research together.
Specifically, they have adopted high-resolution images of an oligo-enediyne (a simple molecule consisting of three benzene rings joined by carbon atoms) on a flat silver surface. The technique used was the non-contact atomic force microscope (nc-AFM). This microscope, with a very thin needle at its end, is capable of detecting even the smallest protuberances on an atomic scale, as can be read words written in braille with fingertips.
To detect protuberances of this scale, the microscope consists of a single carbon monoxide molecule (CO) at the tip of the needle. “The carbon atom is attached to the tip of the microscope and the oxygen atom becomes sensor,” explains Dimas Oteyza, the first author of the article and researcher at CFM. It is a very appropriate sensor because “on the one hand it is small and on the other, it is inert, stable, that is, we can approach a lot to the molecule in study without fear to react with the oxygen atom”, continued Oteyza. The atom vibrates according to what it has under, and they have been able to know according to the frequency of vibration if what it has under is an atom or if the link between the atoms is covalent, as well as the type of covalent bond, that is, if it is simple, double or triple.
Oteyza seems “fascinating” to be able to see the molecules first hand, and he has pointed out that it has been special, among other things, because in student times they have drawn them all in the slate. Now we have been able to see what we have drawn.” Looking at researchers, it also sees it as a tool to better understand chemical reactions and help in molecular identification. “It is an advantage of the molecular ability to work,” he says.
The light looks inside a molecule
In the other research, the highest level of resolution achieved so far with light has been achieved. The management of the research has been carried out by researchers from the Chinese University of Science and Technology (USTC), in which CFM researcher and DIPC Javier Aizpurua participated.
The combination of tunnel effect microscopy and Raman spectroscopy has allowed to represent a single molecule of scale less than 1 nm. “At first we did not expect to achieve such a resolution,” says DIPC researcher Javier Aizpurua. In fact, with the light itself, due to the diffraction limit, it is not possible to separate objects under 200 nm. “Raman spectroscopy is a very special technology that allowed to separate objects up to 10 nm. We, however, have improved this level of resolution ten times.”
They had to work in very special conditions to achieve a resolution of 1 nm: The tunnel effect microscope has been installed in ultra-fast and low-temperature vacuum conditions, combining it with the large Raman surface spectroscopy. “The sum of both produces a non-linear effect from which we return to receive a signal in such high resolution,” explains Aizpurua. In short, the light instead of hitting directly against the object, strikes against the microscope tip and produces a vibration in the molecule below. Well, by synchronizing this vibration with the vibration of the microscope's extreme electrons, an optical signal can be obtained with a resolution lower than a nanometer.
This level of resolution has only been possible to date by using electrons as a probe. “However, with the current technique, in addition to the high-resolution image, we can know which molecules we are representing. Because of its chemical structure, the molecules vibrate in one way or another,” explains Aizpurua.





