Bacteria also have skeleton
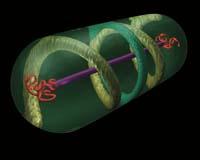
Perritos confusos. Until recently the bacteria were in the eyes of biologists: sacks containing genetic material, some enzymes and other mixed molecules; no stable structures, no order, no skeletons. But things are changing. Within these sacks various structures have been found. Many of them are equivalent to the cytoskeleton components of eukaryotic cells.
In eukaryotic cells, that is, of plants, animals, fungi and protists, the inner network called cytoskeleton is well known. It is a dynamic structure, composed of three types of components: microfilaments (actin), medium filaments and microtubules (tubulin). And it fulfills several functions: giving and maintaining shape to cells, facilitating movement, intracellular transport and cell division...
It was considered that the bacteria had no cytoskeleton, so it was considered that it was developed after the formation of eukaryotes (from bacteria). But now it seems that it was not: the origin of actins, medium filaments and tubulin could be in bacteria.
The bacteria have gone from being secondary protagonists to being fashionable in the field of cell biology, and the discoveries happen.
Behind the shapes

It can be said that in 2001 the wheel of discoveries took the rhythm. In that year, a group of researchers from the University of Newcastle in England first managed to see the structure of the MreB protein within the bacterium Bacillus subtilis: MreB fibers form a propeller under the wall of cylindrical bacteria.
The MreB protein is very similar to the actin of eukaryotes and may have the same origin. In addition, they fulfill a similar function. In cylindrical bacteria the MreB proteins and very close to it have a structural function, that is, they give shape to the bacteria.
In fact, researchers have seen that if they lack this protein changes its appearance. B. subtilis, MreB affects the width of the bacteria and Mbl to length; and E. The coli bacteria go from being cylindrical to spherical when the MreB is removed.
In fact, spherical bacteria do not have MreB. Moreover, analyzing the genomes of various bacteria and arches (the two groups of prokaryotes), Newcastle researchers have found that cylindrical, filamentous and helicoidal prokaryotes contain one or more genes of the MreB protein, while the spherical ones have none.
On the other hand, in more complex forms of certain bacteria, cytoskeleton is very likely to be related. For the moment, they have only found an example: The Caulobacter crescentus bacteria have the form of beans, and it seems that the other protein gives them that curvature. By removing this protein these bacteria acquire cylindrical form. This protein is krescentine and is very similar to the average filament of eukaryotes.
Keys of the division
Apart from the forms, cytoskeleton also has great importance in cell division. And in that the prokaryotes have nothing in common. The bacteria present in the proper environment can be divided every half an hour. As one of the keys to this efficacy can be precisely in the cytoskeleton. But scientists have only begun to clarify the mechanisms.
Soluble Z protein is one of the protagonists of the cell division of prokaryotes. It forms a ring in the area where the division is performed, in the center of the cell. By removing this protein to the cylindrical bacilli, they will stretch and stretch without dividing into two. On the other hand, spherical Staphylococcus aureus, once the gene from Fere Z has been eliminated, begin to build the new cell wall anywhere and can increase the volume eight times before it explodes.
In some ways, the FloridaZ protein controls where the new wall must be built. Around it collects the proteins that build the wall. In the case of S. aureus forms the ring in the equator and governs the creation of the two new hemispheres. In the bacilli, on the other hand, it seems that at first the MreB protein causes the stretching of the cell and then comes into play the Fere Z, creating new rounded ends to divide the cell into two.
GraphicZ rings can have great importance in the origin of cell division. A group of researchers at Duke University in North Carolina have recently seen that this protein also forms rings within microscopic drops of oil. Now, although the cell division of bacteria is a more complex mechanism, researchers believe that the most primitive cells would have enough GraphicZ to divide into two.
On the other hand, some eukaryotic cell organelles, mitochondria and chloroplasts use the Fere Z ring to divide. This reinforces, in short, the belief that these organelles are prokaryotes that in their day were introduced into a eukaryotic cell.
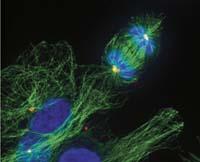
Modified tools Tools
Eukaryotes also have a kind of ForkZ protein, tubulin. And tubulin also participates in cell division, but fulfills a very different function: keeps the chromosomes corresponding to each new cell away during division. This ensures that each new cell will have its chromosomes.
It is curious that these two proteins of the same origin fulfill such a different function. In other words, Fere Z and tubulin are two versions of the same tool that prokaryotes and eukaryotes use to perform very different works. For the same work they use very different tools. On the one hand, for the work of the ForkZ protein, equivalent to tubulin, eukaryotes use an actin tool. On the other hand, prokaryotes do what eukaryotes do with tubulin with a protein equivalent to actin.
And it is that, like eukaryotes, bacteria must also distribute the genetic material well in fragmentation. The bacteria have a single chromosome, in addition to DNA rings called plasma. Plasmids provide resistance to antibiotics and other 'extra' bacteria, so it is very important that the new bacteria stay with a copy of the plasmids.
As tubulins do with the chromosomes of eukaryotes, the protein ParM produces the separation of some plasms, distancing the copies. And that ParM is a bacterial version of actin.

ParM is a protein chain with the ability to self-add and remove units. The ParM chain joins the two new copies of the plasmid to be distributed. Subsequently, by adding new units, the chain stretching begins, which sends the two plasmids to the two ends of the cell.
This is the mechanism of distribution of some plasmids. But what about the chromosome of bacteria? How do copies of the chromosome distribute? That remains a mystery.
And it is not the only one. There is a long way to go to understand well the organization and functioning of the prokaryotic cells. In addition to those already mentioned, they have found many more elements of the cytoskeleton and are found. Also proteins that have nothing in the cytoskeleton of eukaryotes. But we know little about the mechanisms behind all these elements. There is still much to be investigated within these merits.






