From plastic waste to pharmaceutical industry
Plastics are the most abundant synthetic consumer product in the world, reaching an annual production of 390 million metric tons in 2021. Its properties, such as light weight, low cost and easy propulsion, allow a wide variety of applications, such as light packaging, construction, electronics, biomedical devices and energy storage.
At the molecular level, plastics are chains of long monomers, that is, chains created by the repetitions of a molecule, whose properties of the materials depend on their movement and organization. Despite the important advantages that the use of polymers has brought to society, the management of pits has not developed in the same production process, resulting in plastic waste batteries and microplastics.
Although society is raising awareness about unsustainable plastic management and plastic consumption has declined by 10 million tonnes since 2017, it is expected to continue producing a lot of plastic. That is why we have seen the need to manage post-consumer plastics and, as far as possible, give a new life, which is the objective of my doctoral thesis.
Recyclability of polymers
The European Union Environment Committee is seeking to increase the sustainability of commercial plastics by implementing specific policies and programmes. Among these efforts, it aims to transform linear production so far to circular production, enhancing recycling, that is, it recommends the use of plastic waste after consumption as a high quality source for the production of new plastics.
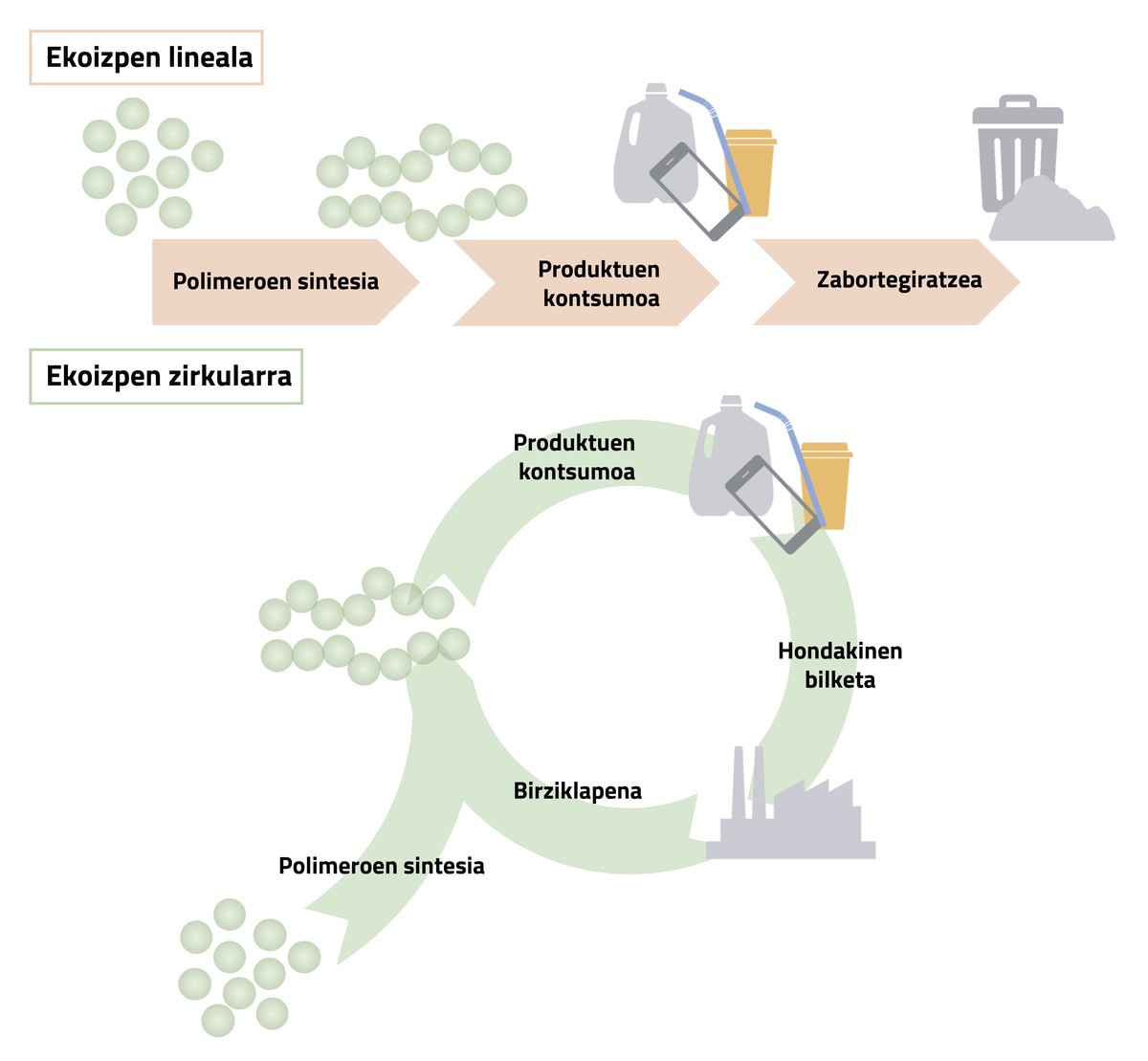
In linear production, for polymer formation, monkeys are taken and polymer synthesis is performed. This polymer is then processed to obtain the desired product, that is, it is given shape and color. Once the product is consumed, the plastic residue is stored in a landfill or used to recover energy (Figure 1).
Circular production is intended to give new life to plastic, making it again a consumer product. In this case, the polymer is first synthesized and processed in the same way as in linear production, but once the polymer is used, the waste is collected and transferred to the specialised industries. In these industries a recycling process is applied to plastic and re-used (Figure 1).
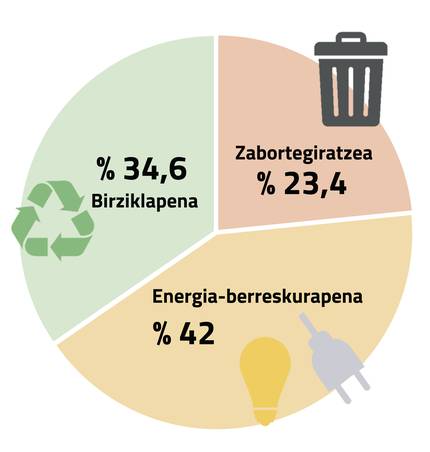
Despite the development of recycling processes, there is still a long way to go, as 23.4% of waste collected in 2020 ended in landfill, 42% was destined for energy recovery and only 34.6% of waste gave a new life (Figure 2). However, it is clear that little by little more is recycled: Since 2006, the use of recycled material has increased by 117% and landfill waste has decreased by 47%.
What do we talk about when we talk about recycling?
Currently, the most common recycling process for plastic waste is mechanical reconversion. However, in this process, the polymer loses properties. Mechanical recycling therefore only supports specific cycles, so plastic cannot be recycled in this way.
Fortunately, it is possible to give plastics an infinite recycling cycle, replacing mechanical recycling by chemical recycling. This recycling method allows the reconversion of polymer chains into monomers, allowing a new synthesis of the initial polymer. Recycled plastic can therefore be produced over and over with the same properties, reducing the need to use new raw materials.
Where do polymers come from?
Most industrial polymers are petroleum based, raw materials are refined from oil and used for polymerization. Drastic conditions such as high temperatures and pressures are used in the refining process and methane and carbon dioxide responsible for climate change are released into the atmosphere.
In order to reduce these damage, the industrial use of biologically based polymers has begun to increase. One of the best known polymers is polylactic acid (PLA), which is sometimes used to pack food. The monomers of this polymer are obtained from plants, which helps to reduce the use of oil. Together with the PLA polymer, in recent years there is another biobased polymer that is acquiring interest: polyhydroxybutyrate (PHB).
Why is polyhydroxybutyrate so special?
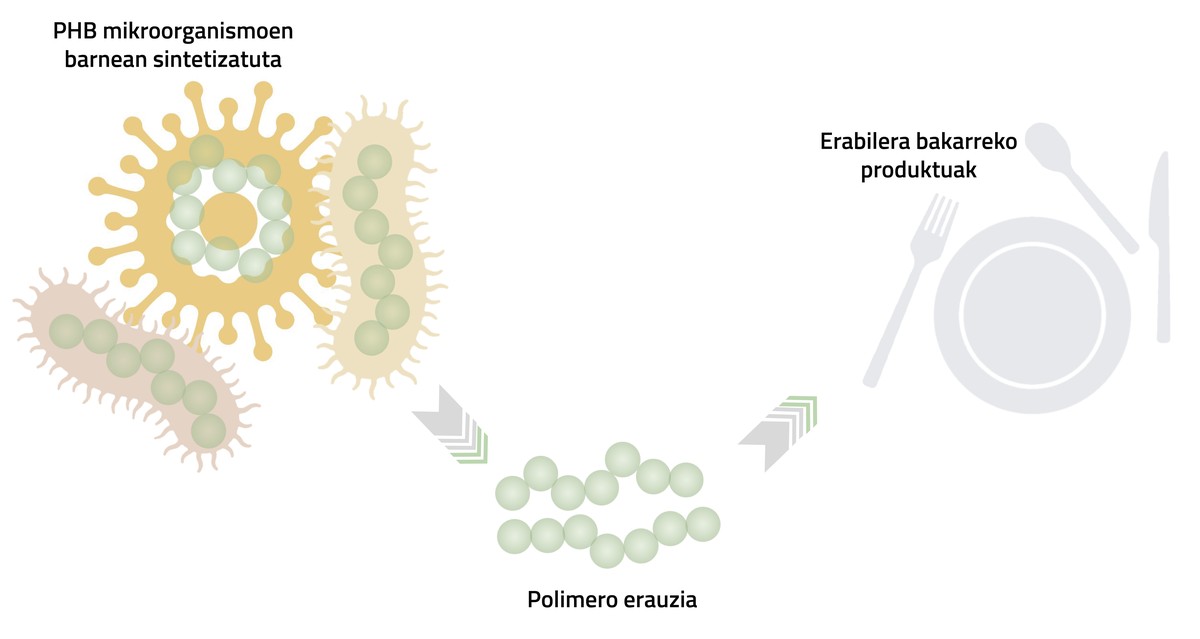
PHB polymer has excellent barrier properties, that is, it does not allow filter small molecules through the polymer, so it is a very suitable material for food conservation. However, the main peculiarity of this polymer lies in its origin, as it is produced internally by microorganisms. Consequently, the polymer is extracted from micro-organisms and used to produce products such as single-use cutlery and light food containers (Figure 3).
This polymer is also biodegradable, which increases interest in it, as it will eventually return to nature in the form of water, carbon dioxide and biomass. However, the main drawback of PHB is its high price, higher than that of conventional oil-based polymers. Therefore, the need for PHB collection has been seen to be a cost-effective polymer for single-use applications.
PHB plastic cannot be recycled mechanically as it degrades to the temperature required by the process and would also lose the products during the process. So we've stressed that the best recycling process for this polymer is chemical recycling.
Chemical recycling of polyhydroxybutyrate
Depending on the polymer, chemical recycling can be done in one way or another, but we've seen that the most appropriate thing is hydrolysis. In the hydrolysis process, water is used to fragment the polymer and thus obtain monomers.
The ideal for hydrolysis of the PHB polymer is to immerse it and raise the temperature to 180ºC, as we have seen. A natural catalyst has been added to assist in the process and has been allowed to react for 12 hours. After this time, the product has been studied by a technique called nuclear magnetic resonance imaging and the monomer obtained is 3-hydroxybutyric acid.
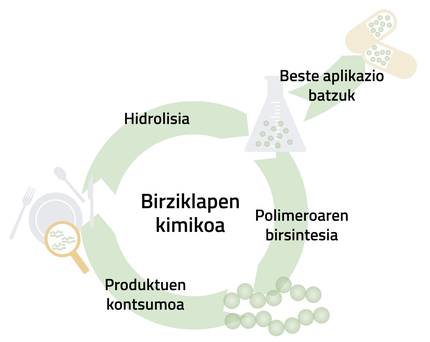
The 3-hydroxybutyric acid molecule offers several recycling pathways. On the one hand, it allows the PHB polymer to be synthesized again, thus closing the recycling cycle completely. However, there are other applications of greater interest, especially those related to the cosmetic and pharmaceutical industries (Figure 4).
In cosmetics, 3-hydroxybutyric acid is used for the treatment of certain skin diseases, such as photoaging, acne, pigmentation disorders and psoriasis. It is also the raw material for the synthesis of numerous compounds containing pharmaceutical acid, such as antitumor agents, anti-obesity agents, antibiotics and vitamins.
3-hydroxybutyric acid can also be obtained by other routes, but these are very difficult processes and very small amounts of product are obtained. It is therefore concluded that PHB polymer hydrolysis is an easy and sustainable way to get this interesting molecule. In addition, it allows nature to be preserved, since from a plastic residue a very important raw material is obtained.