LCD technology turns 40: a story as unknown as it is curious
This year the 5CB molecule needed for the development of LCDs is 40 years old. The discovery is due to the Scottish George Gray and his two colleagues from the University of Hull. We are surrounded by clocks, calculators and televisions equipped with liquid crystal display screens, a molecule that has influenced our society more than any discovery developed by the Nobel Laureates of the last 40 years. Thanks to this molecule, this article can be read on a tablet, phone or monitor and a molecule has become a millennial business. According to the Eureka UK list, it is one of the 100 most important discoveries in the UK that have changed the world.
Liquid crystals
Several articles have been published on liquid crystals in Basque (A. Santamaría, Elhuyar, 1989; M. Iriarte, Berria, 2009). In short, liquid crystal is a physical state between solid state and liquid. It has the ability to flow like a liquid, and is able to maintain order, on one or another plane, when the temperature increases from solid state to liquid. In solid state, all molecules are compact in a three-dimensional structure and do not move until they reach a certain temperature. Then the molecules are freer and begin to move. The system collapses and the solid transforms into mixed liquid. However, since the shape of the crystals is "in the form of a cane", if heated, they suffer a rotation movement; despite losing the order of the crystals, all remain parallel and have characteristics of their own between solid and liquid. Therefore, although the name suggests otherwise, they are neither liquid nor solid.
Friedrich Reinitzer, Czech, first discovered liquid crystals in 1888. When researching cholesterol benzoate, he found that it had two melting temperatures. The solid cholesterol extracted from the carrot was transformed into white liquid at 145.5°C and totally transparent liquid at 178.5°C. After a year, Otto Lehmann realises that he is facing a new state of materials between solids and liquids, and when he shows characteristics of both liquids and solids, he is called liquid crystal. However, until 1911 its structure and characteristics were not well studied, and although during the next five decades it was investigated in numerous European universities, no clear applications were found for this compound.
Cyanobiphenyl discovery
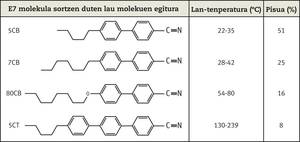
One of them was the University of Hull, dedicated to liquid crystal research. In 1947 George Gray went to perform his thesis at Hull, where he studied liquid crystals for more than 40 years. Gray and her small group triumphed with the synthesis of a molecule, the 4-cyano-4´-pentildifenil (5CB). Previously other compounds were synthesized (ester carbonates, stilbenes, etc.) and were tested on screens, but they were not successful. Often, to obtain new molecules, new groups are added to known molecules. In this case, it was decided to eliminate the functional groups of the initial molecules, that is, to simplify the molecules. Unlike other similar molecules used in LCD until 1972, the 5CB molecule was stable between 22 and 35°C. This was essential to use at room temperature. It is clear, however, that the temperature range of 22-35ºC is not enough to use LCDs in most applications, so the 5CB molecule was mixed with other molecules to obtain the E7 molecule. This molecule could be used at much higher temperature intervals. The discovery of 5CB and E7 was published in 1973 in an article that has become a classic. The demand for this molecule was enormous and the Hull Liquid Crystals Group was unable to respond to that demand. Thus, the company BDH with which it collaborated, Merck currently obtained the contract of sale of this molecule.
As in many other discoveries, the path to the synthesis of this molecule was hard, especially because of the low funding of this field of liquid crystals without apparent clear applications. And for research on liquid crystals to be out of a curious and unimportant subject, and to reach that ubiquity it has today, there were two keys: the need to make new types of screens and the suspicions of a strange British minister of the time.
The Minister of Technology, John Stonehouse, wanted to develop a new technology to build colored flat screens that would replace CRT televisions. On one occasion, the director of the Radar Research Establishment (RRE) acknowledged that the amount the UK was paying to the US. by royalties of this type of television was superior to that which was investing in the development of the Concerto. The day after his hearing, early in the morning, without wasting time, the minister launched a new program to replace the CRT. Therefore, in 1968 a group of military and scientists were launched to develop these new flat screens as soon as possible. This group proposed technologies and research areas such as liquid crystal technology.
At the first meeting held by the Liquid Crystals team, 27 experts from the UK and several generals and admirals met. After several debates, the prestigious physicist Cyril Hilsum asked his colleagues about liquid crystals: Why did light produce, after crossing a sample of liquid crystals, that peculiar structure they saw in the projector? Nobody knew how to respond and, after a long and shameful silence, a voice answered from the last class chairs: "I may help." Needless to say, that voice was from George Gray and, as soon as he left that meeting, he got a contract to develop a stable liquid crystal at room temperature.
Gray wrote the first English textbook on liquid crystals, but it was not very popular among the chemists of the time. Thus, new liquid crystals were developed, which were patented and published in 1973. After a year, the first LCD displays with 5CB were already on the market, and in the coming years Gray synthesized the molecule as part of more than 90% of digital clocks, calculators and LCD alarm clocks worldwide.
Minister Stonehouse's predictions were fundamental in predicting the importance of liquid crystals, but his later personal life and business went down. As soon as LCDs are first marketed, their clothes appeared on a Florida beach, but no remains of the corpse were found. He falsified his death and traveled with his lover to Australia. There he mingled with the murderous aristocrat Lord Lucan and was arrested. In addition, it was known that for more than ten years since 1960, at the beginning of LCD technology, he worked as a government spy in Czechoslovakia.
Hull University and Patents
Gray got the most important liquid crystals in Hull, but neither the chemist nor the university were enriched at all. What happened to all the money obtained by patents at the time of marketing LCDs? Funding for the project came from the Ministry of Defense, which received much to pay royalties on television with cathodic ray tubes. At the same time, the University of Hull, like other universities, did not think that patent and intellectual property belonged to it, and dedicated only a little money to Gray's research team so that the Ministry of Defense would continue to fund the liquid crystal research team until the patent term was exhausted. At that time, LCDs were patented by the Swiss company Hoffman-LaRoche, and the most outstanding benefits obtained by the University of Hull were published articles and international recognition. Why did the University of Hull not patent this new molecule, supported its discovery and made it the richest university in the world? Richer than Harvard, Oxford or any other? This question is still alive, especially because many current universities, such as the University of the Basque Country, are patenting products and have an office for it.
The complexity of these issues makes it necessary to take into account some considerations:
· Most of the discoveries made by the universities are made with public funding, that is, with taxpayer money, and it would not be easy for us to understand if all this ended up in the pockets of private companies.
· Universities would prefer that their researchers' research have a direct application and be marketed. In this way, what they do would have greater social recognition.
· Universities seek to encourage their peers to participate in great discoveries.
· Companies often seek to work with universities to help them develop their products by financing many projects.
All these interests are often contradictory and are not easily channeled. Perhaps, when it comes to protecting the molecule E7, not only that, but other things that have not been told. So, in this story the British are the most critical. Gray's discovery could leave billions of benefits in the UK, for example if the German company Merck (then BDH) had not bought for £60 million. Although it seemed incredible in the LCD business, there was no company in the UK or Europe dedicated to screen manufacturing. However, Gray was not very worried and, although it was not in Europe, the exploitation of the find in Japan and the Far East was very satisfactory. For Gray, his discovery had three main consequences:
· Launch of new cyanobiphenyl.
· Continue funding of the research team by the Ministry of Defense.
· Move from three to the Hull group to over 20. In addition, as senior researchers such as John Goodby and Stephen Kelly, who I met in Hull, the Hull Liquid Crystals team remains one of the most important groups in the world. Goodby has published more than 400 articles and Kelly is one of three chemists who have synthesized more than 3,000 different liquid crystals (holds 75 patents).
George Gray won numerous awards throughout his life, including: In 1979, Queen´s Award, received in technological matters, and undoubtedly the most important, the 1995 Kyoto Prize, Japanese equivalent to the Nobel Prizes, awarded by the Inamori Foundation. In his speech at the event he reflected on the research carried out throughout his life:
· Success and rapid marketing is due to the development of such materials at any given time. Two years earlier it would not be necessary and two years later other researchers would propose new materials.
· The synthesis of these cyanobiphenols took place in 1972, and were sold just over a year later. Great record! And that record would not have been possible without close collaboration between the university and BDH Ltd.
· In order to achieve the achievements at this level, in addition to the hard day to day, training and previous education is essential. In addition, it takes a little luck and you don't have to get away from opportunities.
The history of LCDs is a history of hard work in which desperations were multiple, but above all it has been the result of competition and collaboration between Europe, the USA and Japan. Each industrial area has contributed differently and wisely: The United States put on the table the ideas and viability of technology, Europe the synthesis of basic science and necessary materials, and Japan the circular implementation of the process and serial production of LCDs. Thus, liquid crystals, which over the 80 years only caused curiosity, and the technology that surrounds them, have created in the next 40 years an industry of 50 billion dollars. Last year 750 million LCD products were sold, and today there are more LCD tools than the number of people we live around the world.