CRISPR, genome editing technique: benefits and concerns
“I still don’t use it, but I’ll probably start this year,” says UPV genetic José Antonio Rodríguez Pérez. Rodríguez wants to use the CRISPR technique to investigate a leukaemia mutation. To study the effect of this mutation, you need mutation cells. “So far the only option was to take cells from patients, but that is very difficult. It is much easier to put that mutation into the cells we have in the laboratory.” You can do this with CRISPR.

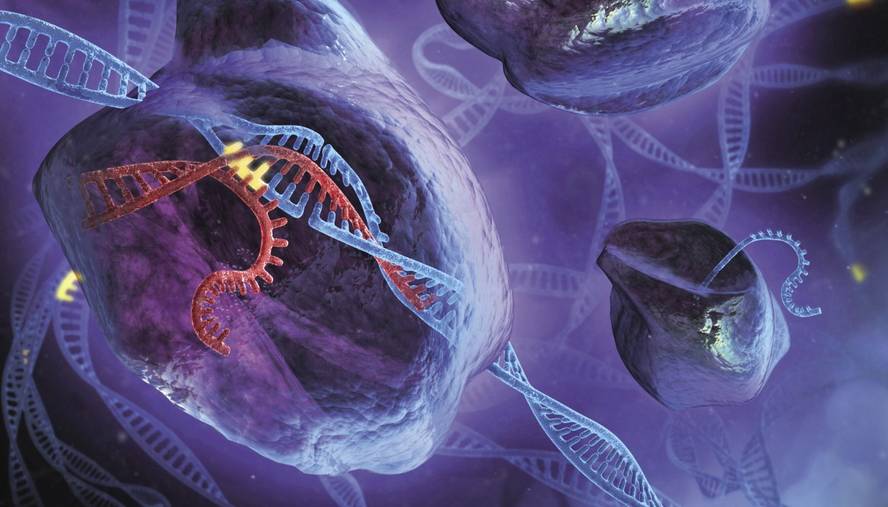
The CRISPR technique is used to edit DNA with great precision. Fragments of DNA can be removed where desired and replaced by new ones. That's not new, there were already other techniques to do so. “The most important thing is that it has simplified and greatly embraced things,” Rodríguez said.
Other instruments very similar to CRISPR, such as TALEN and ZFN. “These devices do the same as CRISPR, but to use them you have to design and assemble a protein that is very expensive. With CRISPR you must design an RNA molecule that is very cheap. Suppose that 100 euros are needed for this, because with the previous techniques thousands of euros would be needed.”
Democratization of democracy
Facilitating both things opens up many possibilities. “You can try different things. Many projects are based on tests and if you have to invest 5,000 euros, you will not start the same, but if they are 200 euros you can try it,” explains Rodríguez. In addition, “he has democratized these experiments, which can be carried out by many people who before could not. Before we could not do this type of small laboratory projects, but now with CRISPR. That is why it has spread so quickly and in this exponential way.”
In 2012 it was published Sciencen Jennifer Doudna and Emmanuelle Charpentier that the enzymatic complex CRISPR-Cas9 of bacteria could be used as a tool of genetic editing. This complex is formed by a RNA guide and the enzyme Cas9. The enzyme cuts DNA at the place given by the RNA guide. In this way you can edit the genome where you want, synthesizing the complementary RNA to the place.
It offers enormous basic research possibilities and its applications can be multiple. Gene therapy is one of the most encouraging. “A giant step has been taken,” says the genetics of the UPV Ana Aguirre Escobal. “Until now, with gene therapy, besides being very expensive, very few good results were obtained, because the technique was not good. Now, however, these results are expected to improve a lot.”
Rodríguez agrees: “In some diseases it can be very important. In many monogenic diseases the gene and defects are well identified and how to correct them. But so far a gene could not be corrected. If a gene was wrong, another direct copy of that gene was introduced.” This copy was introduced through a vector and randomly inserted into the genome, and it had to be expected that the gene would fulfill its function. Now you can enter the correct copy in the place corresponding to that gene, eliminating the previous one. “Now the genes can be corrected in truth,” says Rodríguez.
Working the way to therapy
In 2014, MIT researchers used the CRISPR technique for the first time to treat an illness in adult animals. It was used in the mouse to correct the mutation that produces hepatic disease called thyrosinemia in humans. They managed to cure evil, but at the same time, this work showed that the technique also had some problems to solve.
On the one hand, the technique used to introduce the enzyme Cas9 and the RNA guide in liver cells, the high-pressure injection, is not adequate to be applied in humans. On the other hand, only in one of the 250 cells managed to correct the mutation. In this case, it was enough to cure the disease, but it is very likely that it is not in many other diseases.
On the other hand, “CRISPR still has unspecific effects,” adds Rodríguez, sometimes RNA can be associated elsewhere in the genome. The technique is still optimized, but there is the possibility of improving it. For example, so far the Cas-9 enzyme has been used, but others are testing and can be more specific.”
The MIT team that managed to cure thyrosinemia in mice has already managed to improve the technique. In February, a safer and more effective way to reach the liver cells of mice was published. The RNA guide and the direct fragment of DNA were introduced by a virus and a messenger RNA encoding the Cas9 enzyme by nanoparticles. Thus, in one of every 16 cells the correction was achieved. And they barely detected non-specific effects.
There are more examples, for example, that in December three research groups showed in articles published simultaneously in Science that in the mouse managed to improve Duchenne muscular dystrophy. The issue is going fast, but Rodríguez believes it is still going to take time to apply it to humans: “I don’t think we’re in a position to do so next year, but within a few years I think it will be possible to make changes with great precision in humans. Then we will have to check whether it is allowed or how it is controlled.”
Concerns about the table
In fact, as the capacity of the technique has become clear its amount, and in view of the speed with which it is spreading, the ethical concerns have also increased. “Simplicity is the positive part of this technique, but also the negative, since “playing” with this tool is easy,” Rodríguez said. “I see above all positive aspects. And the risks of the technique are there, but those of other techniques are like long ago.”
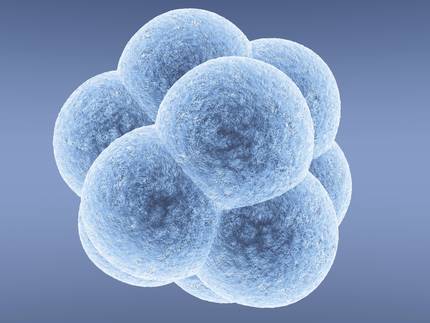
Others, for their part, give greater importance to risks. In March 2015, a group of experts opposed CRISPR and other techniques for the transformation of human embryos in an article published in the journal Nature. These experts believed this could happen shortly. And a month later, researchers from the University of Sun Yats in China published the transformation of human embryos through the CRISPR technique. They used embryos that could not develop, but the experiment ended with the already existing concerns.
Faced with this situation, the American academies of science and medicine, the Royal Society of London and the Chinese Academy of Science organized a meeting to discuss these concerns. In December, 500 experts from around the world met in Washington and reached a provisional agreement: not to ban the publication of the human genome of research, but the use of modified embryos for pregnancies.

“A good thing about science is that everything is very public. We speak and listen and make decisions based on what is happening,” says Aguirre. Aguirre is former chairman of the Research Ethics Committee on Biological Affairs and Genetically Modified Organisms of the UPV/EHU, and the debate that has now been generated on the occasion of CRISPR has not been new. “I remember that in the 1970s, when the first genetic engineering techniques appeared, experts gathered in Asilomar, California. They decided to impose a moratorium. We had to wait a few years to verify that the techniques were safe. Now something similar has happened, they have spoken and agreed: ‘Before starting to talk about applications, let’s make kitchens in the laboratory to improve, optimize and make as safe as possible’.
The debate is ancient. “Things that until now have been distant or very theoretical, have now been closer and feasible, which has resurrected an old semi-dead debate in recent years.” In addition, Aguirre considers that there is another factor that has raised concern: “Some have warned that many laws regulating and regulating genetically modified organisms, as written, exclude the CRISPR technique.” In fact, these laws speak of recombination and strange DNA, but there is no such thing in the CRISPR technique. “I have no doubt that these laws will be adapted to regulate the new technique, but it is true that at this time it has not occurred.”
However, its regulation or regulation varies greatly from country to country. In countries like Japan, China and India there are rules that prohibit genetic transformations in human embryos, but have no legal effects. Regulation in Russia and Argentina is very ambiguous.Funding with public money is prohibited in the United States, but the law does not prohibit clinical application. And in Europe, in general, it is prohibited in the clinic and limited to research. Recently in the UK researchers at the Francis Crick Institute have been allowed to use the CRISPR technique to investigate the development of human embryos.
Fears and expectations
The concerns and fears that CRISPR has generated are always. “Basically, with regard to animals and plants, it is necessary to analyze whether these transformations are safe or not, whether the organisms that are generated can pose a risk to the human being or ecosystems,” said Aguirre. “As for the human being, I believe that the concept of individual is added to security issues. When we talk about changing the sequence of DNA we become nervous because it seems to us that somehow it will influence our individuality.”
However, Aguirre considers that in practice the debate goes to another plane: “If we have near a person with genetic disease and there is an opportunity to cure it, then it is what matters to us. I think when you see that you have real applications, that can improve people’s lives, that society will see these techniques better.”
This is what has happened with many scientific advances. “It occurred with assisted fertilisation. At first it was despicable, but little by little society has demanded it, and children born with fertilization already assisted are no longer seen as demons, as the initials were seen.”
However, genetic transformation can have more debatable applications than the cure of diseases, such as the transformation of embryos to improve certain characteristics. However, Aguirre does not see for the moment too many reasons to worry, “Although it looks closer because it can allow the technique, I think it is still a utopia, a theoretical fear”.
In addition, it highlights that control is rigorous: “Any genomic change technique, such as gene therapy, requires going through different committees. In the Spanish state, for example, there is a very demanding national commission and you must obtain your authorization. But also, if you want to carry out tests in a hospital, you must also go through the ethics committees of that hospital and the autonomous community. There is already a structure and, in any case, that control will harden from now on, since I do not think that genetic improvement has social support. Therefore, I don’t think there are reasons for concern.”
Risk of ecosystem transformation
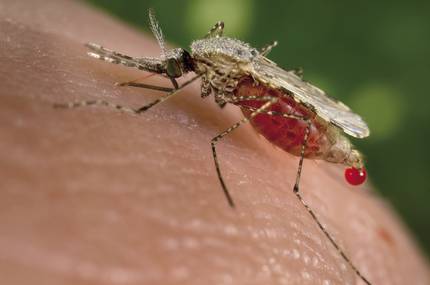
Another concern is the effect that genetically modified living beings can have on ecosystems. And these concerns were raised by another work published in November. Scientists at the University of California created transgenic mosquitoes resistant to the parasite responsible for malaria. In this case, thanks to the CRISPR technique, 99% of the descendants of modified mosquitoes inherited the antibody gene and the gene was active in those mosquitoes. Thus, they estimated that 10 generations would be sufficient to expand into a population.
The capacity of incidence on the population has generated a great concern. Aguirre also relies on the strict controls that exist. “Other transgenic mosquitoes against malaria, made using another technique, have already been released. But for this they had to spend 10 years of controls. Obtaining this type of authorization requires numerous and very deep studies and evaluations that allow knowing the risks and benefits. It is true that in those cases zero risk does not exist. In this case, after putting all the reports on risks and benefits on the table, and taking into account the huge level of malaria, politicians decided to release transgenic mosquitoes. In short, it is a tool that puts science within the reach of society and that it must decide what to do with it.”






